Arsenic (As)-contaminated groundwater used for long-term irrigation has emerged as a serious problem by adding As to soils. Phytoremediation potential of fiber crops viz., kenaf (Hibiscus cannabinus L.), mesta (Hibiscus sabdariffa L.), and jute (Corchorus capsularis L.) was studied to clean up As-contaminated soil.
Varieties of three fiber crops were selected in this study. Seeds of kenaf, mesta, and jute varieties were germinated in As-contaminated soil. Uptake of As by shoot was significantly higher than that by root in the contaminated soil. In As-contaminated soil, kenaf and mesta varieties accumulated more As, than did jute varieties. In the plant parts above ground, mainly the shoots, the highest As absorption was recorded in kenaf cv. HC-3, followed by kenaf cv. HC-95. Kenaf varieties produced more biomass. In terms of higher plant biomass production, and As absorption, kenaf varieties showed considerable potential to remediate As-contaminated soil.
The overall As absorption and phytoremediation potentiality of plant varieties were in the order of kenaf cv. HC-3 > kenaf cv. HC-95 > mesta cv. Samu-93 > jute cv. CVE-3 > jute cv. BJC-7370. All varieties of kenaf, mesta, and jute could be considered for an appropriate green plant-based remediation technology in As-contaminated soil.
Phytoremediation is an effective, low-cost, and promising new method that uses green plants to clean up metal contaminated soils. It is a relatively inexpensive form of ecological engineering that has proven effective. Plants that accumulate metals in high concentrations are sometimes referred to as hyperaccumulators. Hyperaccumulator plants possess highly efficient mechanisms to acquire and concentrate As in their tissues (Ma
Bangladesh has considerable plant biodiversity, and hence, has the potential to provide suitable species for phytoremediation of As-contaminated soil. The problems arising from As contamination in groundwater are major concerns in many countries, especially in Bangladesh, owing to high levels of environmental toxicity to living organisms. Recently, it has become apparent that As-contaminated groundwater used for irrigation is further compounding the problem by adding As to soils, thus posing a serious threat to plants, human health, and environment health, through food chain pathways (Bruce
In order to expand the frontiers of phytoremediation technologies, much focus is now being placed on the increased use of plants that have the potential to accumulate As. There are some problems associated with the application of hyperaccumulators to the contaminated soil, such as small biomass and a limited adaptation capacity to growth conditions and cultivation. The selection of plants having metalaccumulating ability and compatibility with local weather conditions is an important issue. Some plant species such as
>
Experimental site and set up
The net house experiment was carried out in the Department of Agricultural Chemistry, Bangladesh Agricultural University, Mymensingh, located at 24.75°N latitude and 90.4°E longitude. In this investigation, As-contaminated and uncontaminated soils were collected from the selected area of Mymensingh district in Bangladesh, in the agroecological zone (AEZ)-28. Ten kilograms of processed and air-dried soil was taken in a plastic pot (30 cm × 20 cm × 25 cm), and moistened at 70% of the field capacity level, with As-free deionized water. The experiment was performed using a completely randomized design (CRD) with 4 replications. To assess phytoremediation potentiality of kenaf (
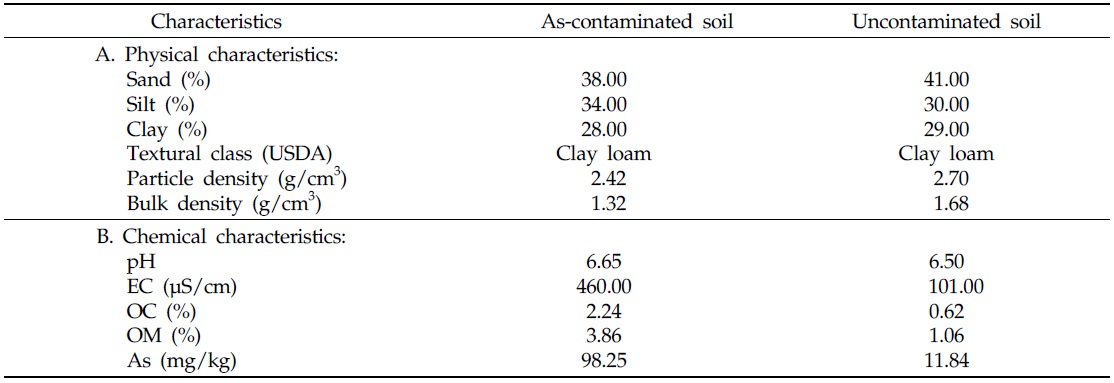
After collection of both contaminated and uncontaminated soils, the physical and chemical characteristics of each soil were recorded (Table 1). Uncontaminated soil typically contains 10.0–12.0 mg/kg As (Smedley and Kinniburgh, 2002; Hossain, 2006; Henke, 2009), therefore, samples falling within this range, in the present study, were considered uncontaminated soil. Soil samples containing 98.25 mg/kg As, were treated as As-contaminated soil. Considering the intensity of soil As contamination, soil samples were collected separately prior to incorporation into each pot. Texture, bulk and particle densities of preharvest soil samples were determined following methods as outlined by Klute (1986). Electrical conductivity (EC) and pH values of preharvest soil samples were measured electrometrically in a 1:2.5 and 1:5 suspension of soil and water, respectively (Singh
[Table 1.] Characteristics of As-contaminated and uncontaminated soils
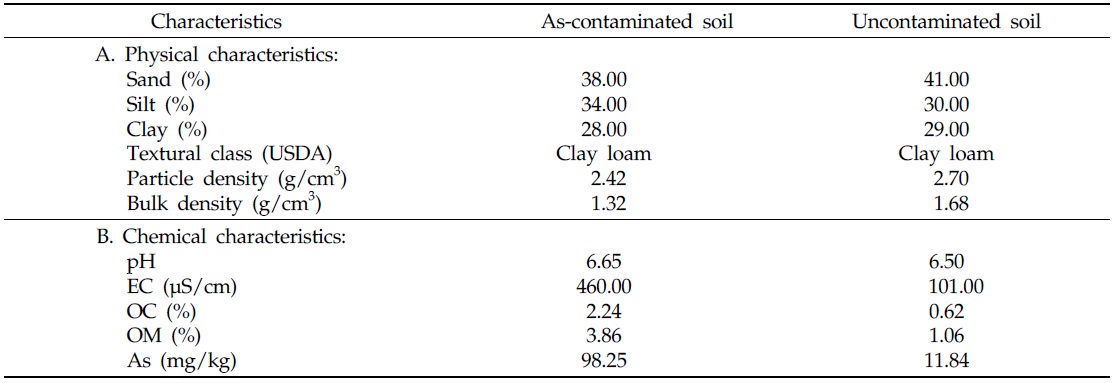
Characteristics of As-contaminated and uncontaminated soils
After collecting plant samples, finely ground plant samples were digested with HNO3 (AR grade), and H2O2 (AR grade), for the determination of As (Cai
Enrichment factor (EF) was calculated to determine the degree of heavy metal accumulation in plants grown on the contaminated soil, in comparison to plants grown on the uncontaminated soil, using the following formula (Kisku
Where,
>
Bioconcentration factor (BCF)
Bioconcentration factor (BCF) provides an index of the ability of the plant to accumulate metal, with respect to metal concentration in the substrate. BCF was calculated from the following formula, as outlined by Ho
Where,
Analysis of experimental data was performed statistically, following the procedure described by Gomez and Gomez (1984). Significance of difference between means was verified by Duncan’s multiple range test (DMRT).
>
Effect of As on plant growth
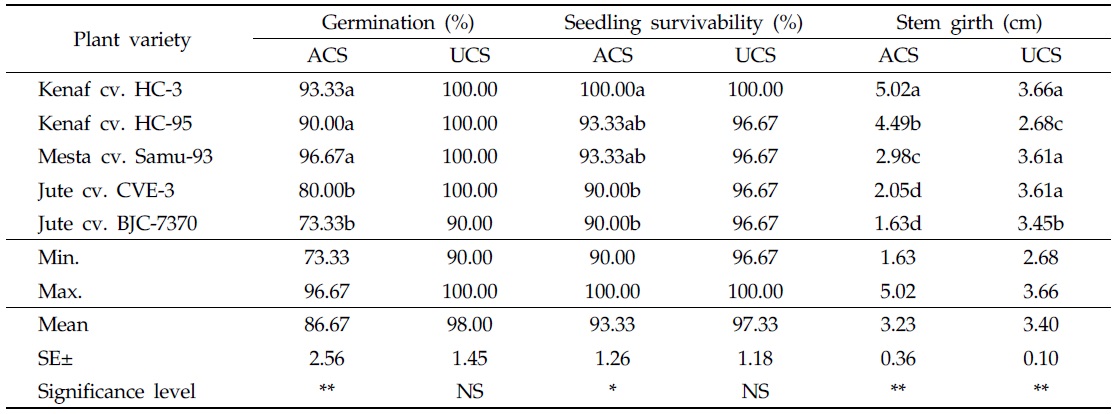
In general, germination of kenaf, mesta, and jute seeds was slightly higher in the uncontaminated soil than in As-contaminated soil. In As-contaminated soil, the highest germination (96.67%) was found in mesta cv. Samu-93, followed by kenaf cv. HC-3 (93.33%), and the lowest germination (73.33%) was recorded in jute cv. BJC-7370. Significant statistical variations existed in germination among all plant varieties; however, plant varieties germinated well on soil contaminated with As. Seed germinations of kenaf cv. HC-95 and mesta cv. Samu-93, were statistically identical. In the uncontaminated soil, maximum germination (100%) was observed in kenaf cv. HC-3 and HC-95, mesta cv. Samu-93, and jute cv. CVE-3, but minimum germination (90%) was recorded in jute cv. BJC-7370 (Table 2). No statistical variations were found in germination of different plant varieties.
[Table 2.] Effect of arsenic on plant growth in As-contaminated and uncontaminated soils
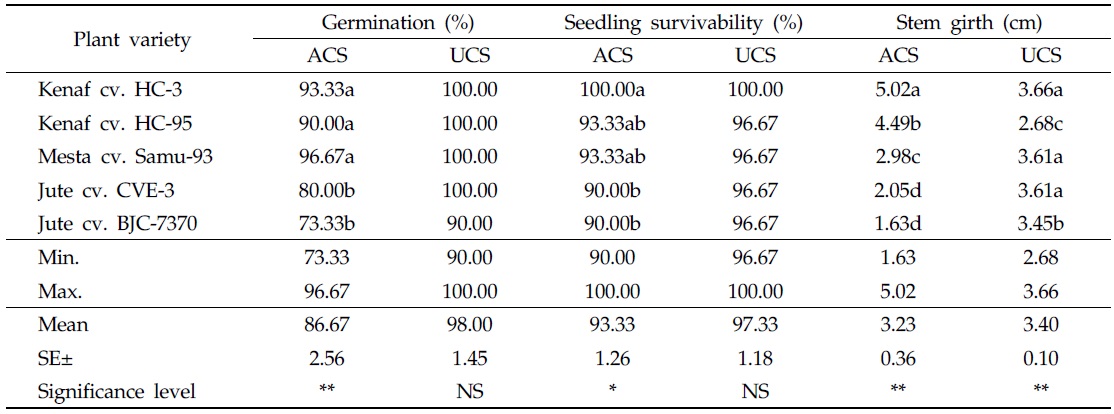
Effect of arsenic on plant growth in As-contaminated and uncontaminated soils
Different plant species have different germination responses in the contaminated soil. The results of the present study, were supported by Mandal and Bhattacharyya (2007), who found that common pulses (
In both As-contaminated and uncontaminated soils, 100% seedling survivability was observed in kenaf cv. HC-3. Other plant varieties like kenaf cv. HC-95, mesta cv. Samu-93, and jute cvs. CVE-3 and BJC-7370 showed 96.67% seedling survivability in the uncontaminated soil. In As-contaminated soil, 93.33% of kenaf cv. HC-95 and mesta cv. Samu-93 seedlings survived. In As-contaminated soil, the lowest seedling survivability (90%) was detected in two jute varieties (Table 2). Seedling survivability is one of the most important characteristics of a plant that absorbs toxic metal from the contaminated soil. Significant effects were observed on seedling survivability in As-contaminated soil; however, the varieties under consideration easily survived in As-contaminated soil containing 98.25 mg/kg As. The observed survivability of the selected varieties of kenaf, mesta, and jute was consistent with the findings of Islam
At 120 DAS, the largest stem girth (5.02 cm) was measured in kenaf cv. HC-3, followed by kenaf cv. HC-95 (4.49 cm), in As-contaminated soil. In the uncontaminated soil, stem girth of different plant varieties was less than that of the same varieties in the contaminated soil. The highest stem girth value (3.66 cm) was found in kenaf cv. HC-3, followed by mesta cv. Samu-93 (3.61 cm) and jute cv. CVE-3 (3.61 cm). This result may be attributed to greater nutrient absorption potential of kenaf, even in As-contaminated conditions. In addition, stem girths of mesta cv. Samu-93 and jute cvs. CVE-3 and BJC-7370 in the uncontaminated soil were higher than those of the same varieties grown in As-contaminated soil (Table 2).
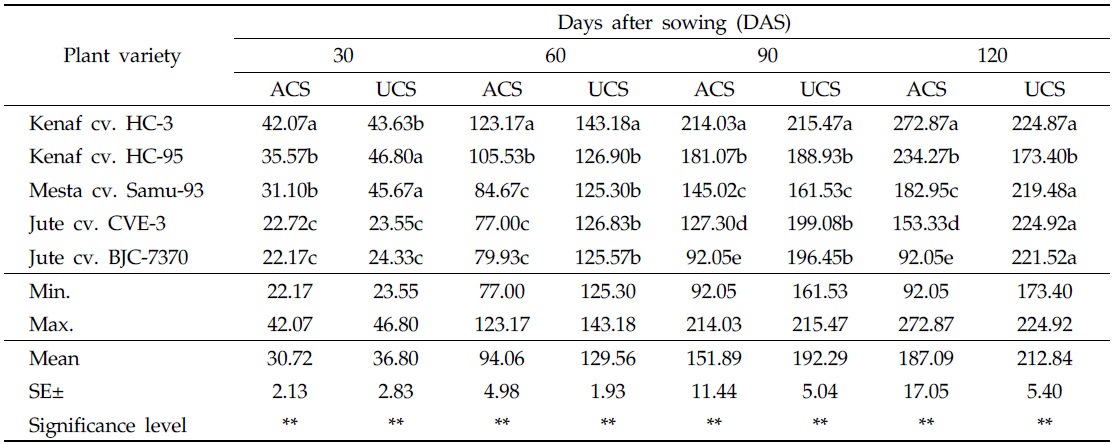
In both uncontaminated and As-contaminated soils, plant heights differed from variety to variety at different DAS. At 30 and 60 DAS, plant heights of all varieties were higher in the uncontaminated soil than in As-contaminated soil. At 30 DAS, maximum plant height (42.07 cm) was recorded for kenaf cv. HC-3, and minimum plant height (22.17 cm) was recorded for jute cv. BJC-7370 in As-contaminated soil. On the other hand, maximum plant height (46.80 cm) was recorded for kenaf cv. HC-95, in the uncontaminated soil, and the minimum value (23.55 cm) was recorded for jute cv. CVE-3 (Table 3). At 60 DAS, maximum plant height (123.17 cm) was recorded for kenaf cv. HC-3, and minimum plant height (77.00 cm) was recorded for jute cv. CVE-3, in As-contaminated soil. The highest value of plant height (143.18 cm) measured in the uncontaminated soil was recorded for kenaf cv. HC-3, and the lowest value (125.30 cm), for mesta cv. Samu-93 (Table 3).
[Table 3.] Effect of arsenic on plant height in As-contaminated and uncontaminated soils
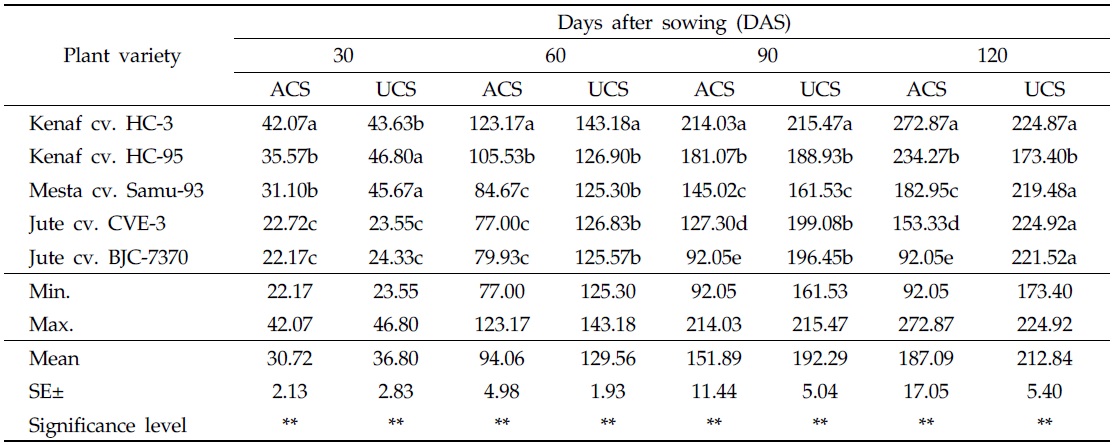
Effect of arsenic on plant height in As-contaminated and uncontaminated soils
At 90 DAS, heights of all plant varieties were higher in the uncontaminated soil than in As-contaminated soil. During this period, maximum plant height (214.03 cm) was recorded in As-contaminated soil for kenaf cv. HC-3, and minimum plant height (92.05 cm) was observed in jute cv. BJC-7370. On the other hand, the highest plant height (215.47 cm) was recorded in the uncontaminated soil from the variety of kenaf cv. HC-3, and the lowest plant height (161.53 cm) was recorded in mesta cv. Samu-93 (Table 3). At 120 DAS or harvesting stage, plant heights of kenaf cvs. HC-3 and HC-95 were higher in As-contaminated soil than in the uncontaminated soil. In this period, maximum plant height (272.87 cm) was recorded in the contaminated soil, from the variety of kenaf cv. HC-3, and minimum plant height (92.05 cm) was recorded in jute cv. BJC-7370. In the uncontaminated soil, maximum plant height (224.92 cm) was recorded in jute CVE-3, and minimum plant height (173.40 cm) was recorded in kenaf cv. HC-95 (Table 3).
The present study related to plant height agreed with the findings of Islam
>
Effect of As on plant biomass production
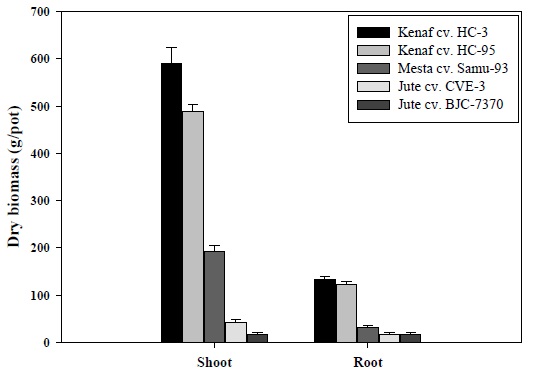
The experimental results in Fig. 1 indicate that dry plant biomass production of roots and shoots varied from variety to variety. In As-contaminated soil, the highest dry biomass (589.39 g/pot) of shoot was measured in kenaf cv. HC-3, followed by kenaf cv. HC-95 (487.23 g/pot), and the lowest biomass (17.20 g/pot) was measured in jute cv. BJC-7370. Maximum dry biomass (133.70 g/pot) of root, was also measured in kenaf cv. HC-3, followed by kenaf cv. HC-95 (121.93 g/pot), and minimum biomass (16.59 g/pot) was obtained for the variety of jute cv. BJC-7370.
In phytoremediation, the uptake capability of toxic metals and biomass production capacity of plant species are very important considerations when cultivation is done on the contaminated soil. Among the plant species, kenaf varieties grown in As-contaminated soil produced a greater amount of shoot biomass, as compared to other varieties (Fig. 1). These results can be attributed to toxic metal tolerance of plant varieties. Meera and Agamuthu (2011) reported that kenaf was found to have higher biomass and subsequently recorded 11% higher bioaccumulation capacity, indicating its suitability for phytoextraction of As in the contaminated soil.
>
As absorption by plant parts
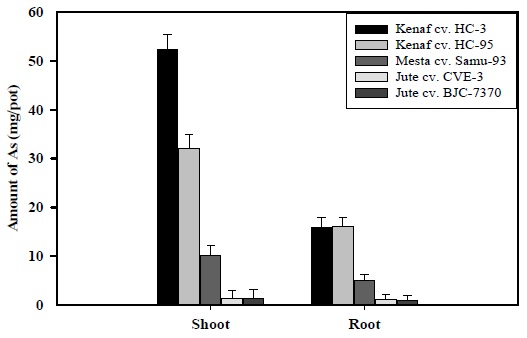
In As-contaminated soil, As absorption by shoots and roots of plant species differed from variety to variety. In As-contaminated soil, maximum amount of As (52.29 mg/pot) was absorbed by shoots of kenaf cv. HC-3, followed by kenaf cv. HC-95 (32.12 mg/pot), whereas minimum amount (1.16 mg/pot) was absorbed by shoot of jute cv. CVE-3 (Fig. 2). In the case of roots, the highest amount of As (16.12 mg/pot) was absorbed by kenaf cv. HC-95, followed by kenaf cv. HC-3 (13.93 mg/pot), and the lowest amount (0.92 mg/pot), was observed in jute cv. BJC-7370 in the contaminated soil. As shown in Fig. 2, maximum As absorption by shoots and roots was found in kenaf varieties grown in the contaminated soil. In the present study, the aboveground plant parts (shoots) absorbed higher amount of As than belowground plant parts (roots), in all the plant varieties.
The present observations were similar to the findings of Sultana and Kobayashi (2011), who concluded that the uptake of As increased in roots and shoots, with increasing levels of As in soil. Other researchers also found that As uptake by plants increased, with increasing As concentration in the growth medium or soil (Ma
>
As accumulation in plant varieties
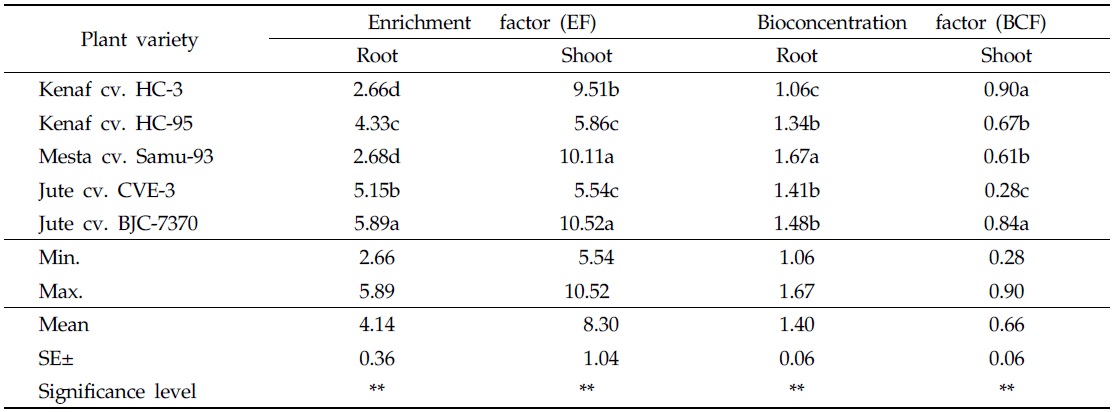
Enrichment factor (EF) was used to evaluate the degree of accumulation of heavy metal in plant parts growing in the contaminated soil, as compared to plants parts growing in the uncontaminated soil. EF of heavy metals in plant parts is a crucial factor in the selection of phytoremediator species growing in heavily-contaminated soils (Barman and Bhargava, 1997). EF values of As for roots and shoots of kenaf, mesta, and jute varieties are presented in Table 4. In the case of EF values in roots, the highest value (5.89) was recorded for the variety of jute cv. BJC-7370 and the lowest value (2.66) was recorded for kenaf cv. HC-3. The highest EF value (10.52) for shoots was measured in the variety of jute cv. BJC-7370 and the lowest EF value (5.54) was recorded for jute cv. CVE-3. Significant variations were found in EF values of roots and shoots among the varieties of kenaf, mesta and jute (Table 4). In all plant varieties, the calculated EF values of roots and shoots were greater than 1. According to Kisku
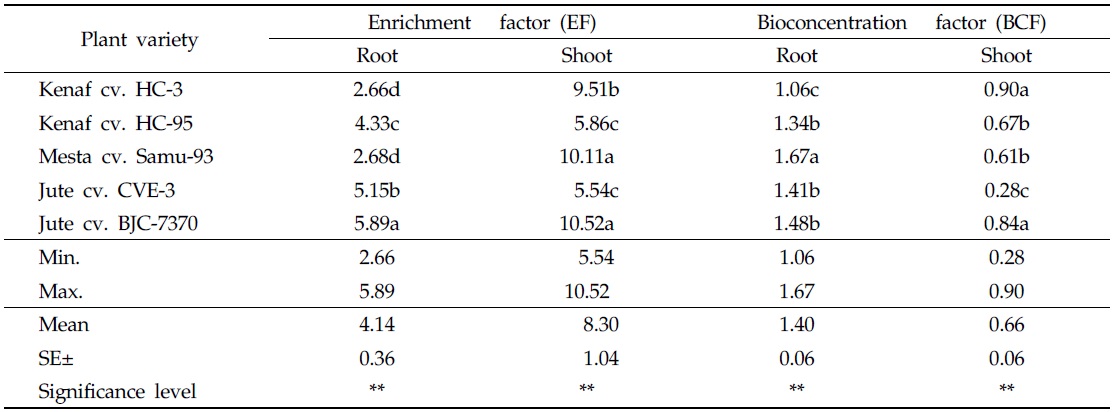
Enrichment factor (EF) and bioconcentration factor (BCF) of arsenic for different plant varieties
Bioconcentration factor (BCF) values of roots and shoots were calculated for As-contaminated soil. The highest BCF value (1.67) for roots was recorded for the variety of mesta cv. Samu-93, and the lowest BCF value (1.06) was recorded for kenaf cv. HC-3 (Table 4). In the case of shoots, the highest BCF value (0.90) was measured in kenaf cv. HC-3 and the lowest BCF value (0.28) was recorded in jute cv. CVE-3 (Table 4). In the case of BCF values in roots and shoots, significant differences existed between the varieties of kenaf, mesta and jute. Bioconcentration factors of roots were higher than that of shoots. Similar trends of observation were reported by Islam
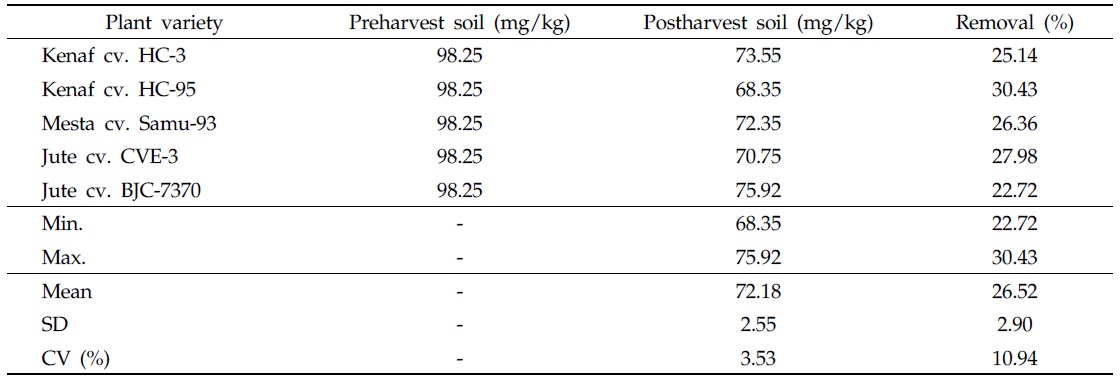
After harvesting plant varieties, the content of As in postharvest soils was found to be less than that in preharvest soils, indicating As removal from soils (Table 5). Removal of As was dependent on plant variety and As status in preharvest soils. In the contaminated soil, the highest As concentration (75.92 mg/kg) was detected in the postharvest soil cultivated with jute cv. BJC-7370, and the lowest As content (68.35 mg/kg) was recorded in the postharvest soil cultivated with kenaf cv. HC-95 (Table 5). Considering As status in the postharvest soil, all plant varieties under study have the capacity to absorb As from the contaminated soil. It is inferred from this investigation that plant varieties under consideration could be used to remediate As-contaminated soil.
[Table 5.] Arsenic level in the contaminated soil
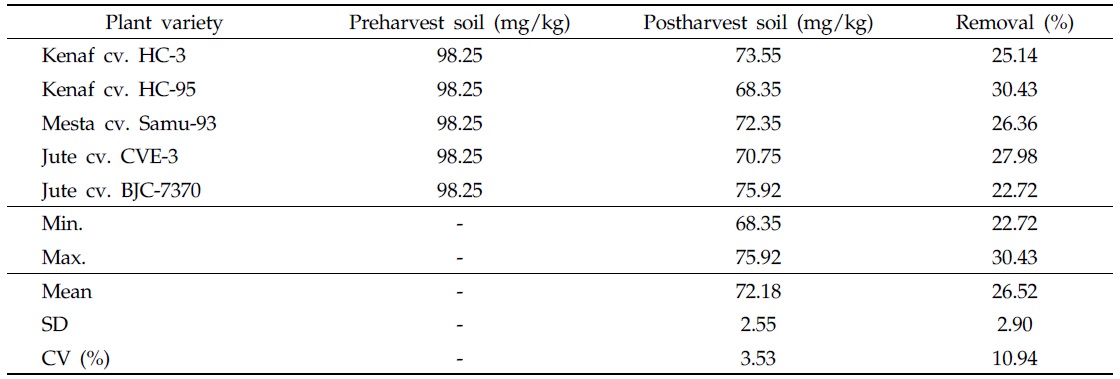
Arsenic level in the contaminated soil
All varieties of kenaf, mesta, and jute were able to germinate in As-contaminated soil, and were hence considered As accumulators, exhibiting remediation capability in the contaminated soil. However, kenaf varieties were more proficient than mesta and jute varieties in removing As from the contaminated soil. The overall As absorption and phytoremediation potentiality of plant varieties were in the order of kenaf cv. HC-3 > kenaf cv. HC-95 > mesta cv. Samu-93 > jute cv. CVE-3 > jute cv. BJC-7370. In conclusion, all varieties of kenaf, mesta, and jute can be considered for phytoremediation technology in As-contaminated soil.