Carbon fibers (CFs) have been used as reinforcements in aircraft, automobiles, boats, high-grade sporting goods, wind turbine blades, gas sensors and electromagnetic shielding due to their exceptionally good mechanical and electrical properties. As a result, demand for CFs has continuously increased, and research on new precursors to satisfy this demand is being conducted [1-3]. In general, the most exploited precursors for the preparation of CFs are pitch and synthetic polymers, but their application is limited due to energy and resource concerns [4-6]. Cellulose can be easily obtained from natural sources [7] and is a precursor to carbon fiber. Additionally, cellulose does not suffer from the energy and resource concerns associated with pitch and synthetic polymers [3]. However, the carbonization yield of cellulose-based carbon fiber does not exceed 10%–15% because of its very low initial carbon content (44.4%). Therefore, the cellulose-to-carbon-fiber yield needs to be as high as possible [8-10].
The preparation process for CFs includes stabilization and carbonization steps. In general, the stabilization process uses thermal stabilization in an air atmosphere. During this process, the chemical structure of the precursor is changed through cyclization, oxidation, dehydrogenation, and cross-linking [11-13]. The modified chemical structure of the precursor affects the carbonization yield of the CFs. However, thermal stabilization is a time-consuming and complex process.
Electron-beam (E-beam) treatment has been shown to induce polymer modification, such as cross-linking, curing, and grafting. The advantages of this method include a short reaction time and a simplified process compared to thermal stabilization [14-17]. In this study, cellulose fibers were treated using E-beam stabilization, which has a short reaction time and a simplified process, and CFs were prepared from these E-beam-stabilized cellulose fibers. The properties of the E-beam-stabilized cellulose fibers were investigated as a function of the absorbed E-beam dose.
2.1. E-beam stabilization and carbonization of cellulose fibers
In this work, the cellulose fiber was a lyocell fiber supplied by KOLON Industries (Korea). Twenty grams of cellulose fibers were treated by E-beam stabilization. During the E-beam stabilization, electrons were accelerated through a voltage gradient of 1.14 MeV, and the absorbed doses from the E-beam stabilization were between 500 and 2000 kGy. As shown in Table 1, the prepared samples were labeled according to the E-beam stabilization dosage conditions, as CeF, ECeF-500, ECeF-1000, ECeF-1500, and ECeF-2000. The E-beam-stabilized cellulose fibers were then carbonized in a nitrogen atmosphere at 800℃ for 1 h at a heating rate of 5℃/min.
[Table 1.] The E-beam stabilization conditions of samples
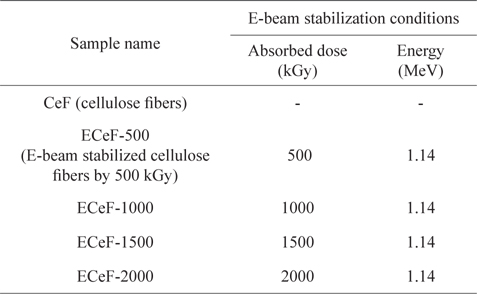
The E-beam stabilization conditions of samples
2.2. Analysis of E-beam-stabilized cellulose fibers
fibers The surface morphology of the samples was observed using scanning electron microscopy (SEM; S-5500, Hitachi, Tokyo, Japan, KBSI) to investigate the effects of the E-beam stabilization on the cellulose fibers. The chemical structures of the samples were investigated using Fourier transform infrared spectroscopy (FT-IR; Bio-Rad Laboratories, Hercules, CA, USA) and X-ray photoelectron spectroscopy (XPS; ThermoVG Scientific, UK). The thermal properties of the samples were investigated using thermogravimetric analysis (TGA; Mettler-Toledo Inc., USA). The TGA scans were performed at 5℃/min under a constant nitrogen gas flow at temperatures ranging from 30℃ to 1000℃.
3.1. Surface properties of the cellulose fibers by E-beam stabilization
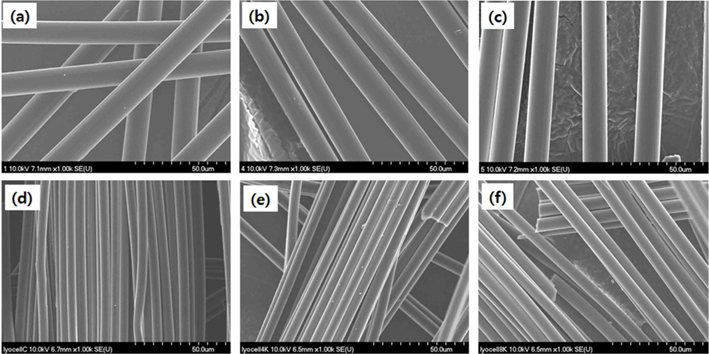
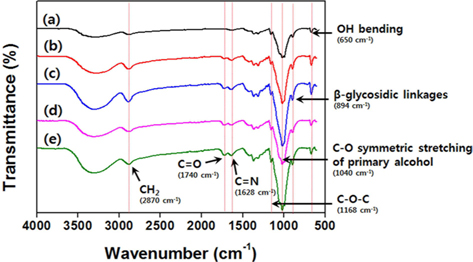
The surface morphology of the E-beam-stabilized cellulose fibers and carbonized cellulose fibers was analyzed using SEM, as shown in Fig. 1. The surface morphologies of the cellulose fibers were unchanged by the E-beam stabilization. However, the cellulose fiber diameter was reduced by carbonization. The surface functional groups on the cellulose fibers exposed to the E-beam were investigated using FT-IR (Fig. 2). The FTIR spectrum shows peaks in the range 1200–500 cm–1 arising from cellulose fiber [18,19]. Pure cellulose fibers do not exhibit C=O (1740 cm–1) [15] or C=N (1628 cm–1) [4] peaks. However, these peaks did appear in the spectra for the E-beam-stabilized cellulose fibers. The oxygen and nitrogen functional groups in the cellulose fibers are modified by the oxygen and nitrogen in the air during the E-beam stabilization [20]. This modification is a result of the electrons created by the electron gun during the E-beam stabilization, forming oxygen and nitrogen radicals from the oxygen and nitrogen in the air. The functional groups of the cellulose fibers were modified by these oxygen and nitrogen radicals. Increases in the absorbed dose during the E-beam stabilization caused the intensity of the C=O, C=N, CH2 (2870 cm–1) and OH peaks (3150–3560 cm–1) in the cellulose fibers to increase.
3.2. Chemical bond structures of the cellulose fibers by E-beam stabilization
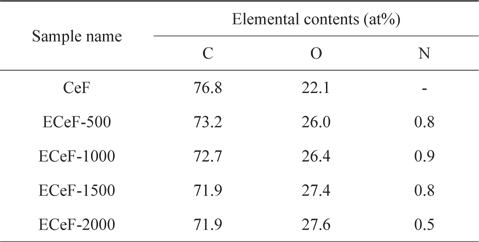
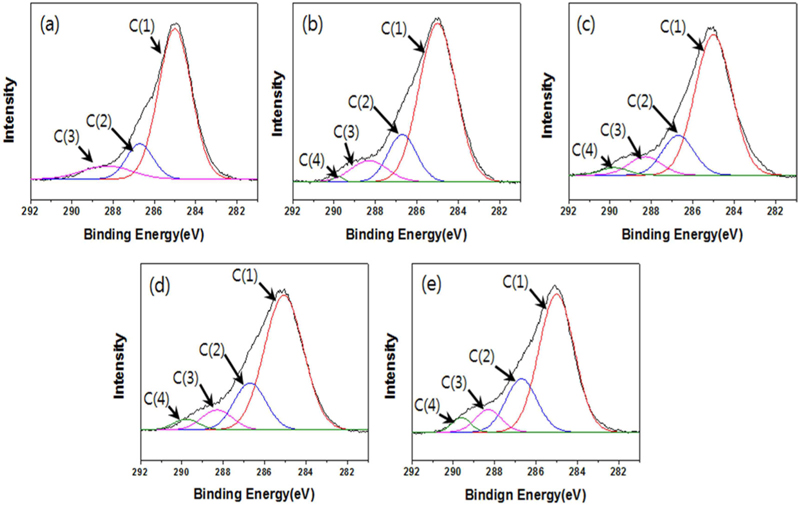
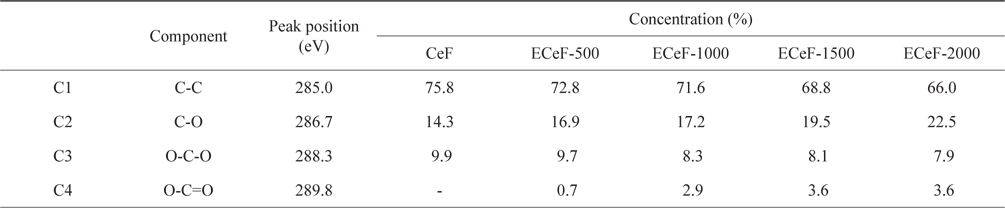
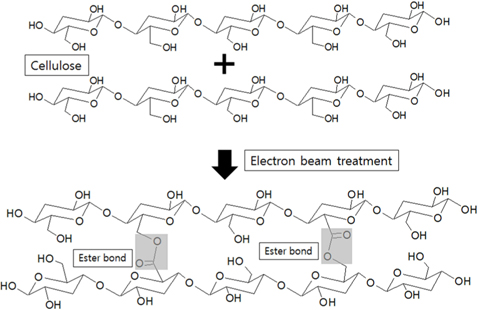
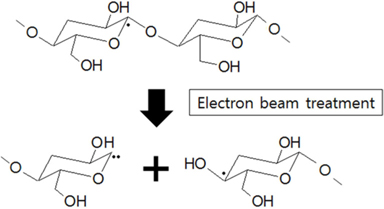
The structures of the surface chemical bonds in the E-beam-stabilized cellulose fibers were analyzed using XPS, and the elemental contents are shown in Table 2. The chemical compositions are also shown in Fig. 3 and Table 3. The surfaces of the E-beam-stabilized cellulose fibers consisted mostly of caron, oxygen and nitrogen. Increasing the absorbed E-beam dose caused the oxygen content to increase and the carbon content to decrease. In contrast to pure cellulose fibers, the E-beam-stabilized cellulose fibers contain an O-C=O bond (289.8 eV, ester bond) [21]. Increasing the absorbed E-beam dose was also found to increase the number of ester bonds in the cellulose fibers. The ECeF-1500 and ECeF-2000 samples exhibited the most ester bonds of the E-beam-stabilized cellulose fibers examined here. The ester bonds were created by reactions between the oxygen radicals and the cellulose fibers, and the ester bonds cross-linked the cellulose fibers, as presented in Fig. 4 [22]. However, the number of O-C-O bonds (288.3 eV) was decreased from 9.9% to 7.9% and the number of C-O bonds (286.7 eV) was increased from 14.3% to 22.5%, which increased with the increase in absorbed E-beam dose. The cellulose fibers transformed to a hemicellulose structure, which consists of short cellulose chains, due to the degradation of the O-C-O bond as a result of the E-beam stabilization (Fig. 5) [23-26].
[Table 2.] XPS surface elemental analysis parameters of samples
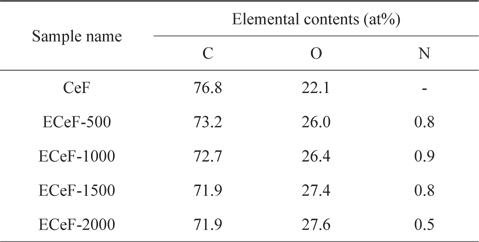
XPS surface elemental analysis parameters of samples
[Table 3.] C1s peak parameters of samples
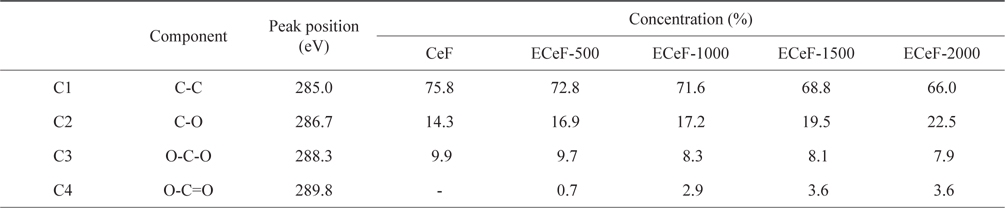
C1s peak parameters of samples
3.3. Thermal properties of the E-beam-stabilized cellulose fibers
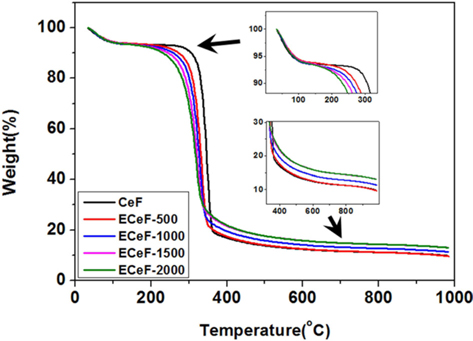
The thermal behavior of the E-beam-stabilized cellulose fibers was investigated by TGA, with the results shown in Fig. 6. The initial degradation temperature (IDT) decreased with an increase in the absorbed E-beam dose. In general, hemicellulose has a low IDT compared to cellulose [21,27]. The XPS results showed that the cellulose fibers were decomposed into hemicellulose by the E-beam stabilization. Therefore, increasing the absorbed E-beam dose decreased the IDT due to the increasing ratio of hemicellulose to cellulose fibers.
At temperatures higher than the IDT, the samples exhibited a significant weight loss as the cellulose degraded. At temperatures over 400℃, all of the samples were carbonized, and the char yield of the cellulose fiber increased with the absorbed E-beam dose. From the XPS results, the cellulose fibers were found to be cross-linked by the E-beam stabilization. The crosslinked degree was increased by the increase in absorbed E-beam dose. In general, cross-linked bonds in polymers increase the degree of the char yield [28-30]. Therefore, the char yield of the cellulose fibers increased with the amount of cross-linked bonds in the cellulose fibers.
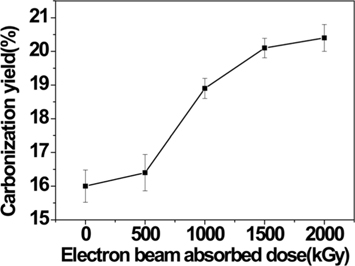
The cellulose fibers and E-beam-stabilized cellulose fibers were carbonized in N2 gas at 800℃ for 1 h. The carbonization yield of the samples is shown Fig. 7. The carbonization yield of the non-treated cellulose fiber was 16%, and electron beam treated cellulose fiber was increased to 20.4%. In this study, the carbonization yield of the cellulose fiber was increased by 27.5% with a 1500 kGy absorbed E-beam dose or greater.
In this work, cellulose fibers were stabilized using an E-beam treatment, and their properties were analyzed with SEM, FT-IR, XPS, and TGA. The E-beam stabilization resulted in decomposition and cross-linking in the cellulose fibers, the degree of which increased with the increase in absorbed E-beam dose. The cellulose decomposed to hemicellulose, which exhibited a decreased IDT However, cross-linking was introduced via ester bonds in the cellulose fibers, which resulted in a 27.5% increase in the carbonization yield for the cellulose-based carbon fiber, compared to the pure cellulose fibers, when stabilized by a 1500 kGy absorbed E-beam dose.