



A number of fluorescence imaging techniques for use in the surgical removal of glioma have been developed over the course of the long history of neurosurgery. Various biomarkers, biochemical agents, and detection systems for glioma have also been developed. This review focuses on 5-aminolevulinic acid (5-ALA), which is used to detect glioma. Numerous forms of fluorescence-guided surgery use 5-ALA, which is helpful to the surgeon. The surgical microscope system is the observational method generally used with 5-ALA, while the loupe, endoscope, and exoscope are simpler alternatives. A system is needed for minimal resection and other issues that arise during neurosurgery. Such an enhanced system should be able to detect low-grade tumors and provide information on microinvasive diseases, resulting in an improved survival rate and better surgical skills. Development of systems that fulfill certain needs would help protect the brain function of the patient and broaden the use of such systems in neurosurgery.
Recently, several groups have studied fluorescence imaging systems and fluorescence biomarkers for clinical application and many preclinical and clinical studies have shown the possibility of fluorescence-guided surgery [1, 2]. Some fluorescence dyes used in such surgery are 5-aminolevulinic acid (5-ALA), fluorescein isothiocyanate (FITC), and indocyanine green (ICG).
Most studies on 5-ALA have been phase III clinical trials, which are randomized controlled multicenter trials. These phase III clinical trials have shown that the use of 5-ALA in surgery produces reliable results. This surgical method is more convenient for tumor resection and enables better resection of the tumor boundary, which improves survival rate [3-7].
5-ALA is not a fluorescent, but when 5-ALA fluorescent dye is taken orally, metabolism converts it to protoporphyrin IX (PpIX). The PpIX in cells is excited by 400 nm wavelength light, causing it to emit 635 nm wavelength light, which enables detection of the position of a tumor [8].
Since 1975, ICG has been an effective fluorescent used to detect the position of blood vessels with few side effects. Upon injection, ICG combines with plasma proteins. It has an excitation wavelength of 750-800 nm and emits 845 nm wavelength light [9, 10]. Several methods have been used to label tumors, including tattooing with India ink or ICG, intraoperative colonoscopy, and resection with endoscopic clips [11].
Glioma, one of the most common primary brain tumors, shows malignant progression characterized by early widespread invasion throughout the brain. Even low-grade tumors show early diffuse infiltration into the surrounding area, which makes them incurable via surgical means. Invasion of glioma into normal brain tissue is a major challenge to clinical intervention because total surgical resection is impossible and any resection eventually leads to recurrent tumors [12]. The best treatment for glioblastoma multiforme (GBM), the most common malignant type of glioma, is extensive surgical resection, when possible, accompanied by chemotherapy and radiotherapy. However, the survival of patients with GBM is <2 years. Many papers have reported that the extent of resection of GBM is the primary determinant of the length of life expectancy [13-16]. However, it is not easy to totally remove glioma because the interface between the tumor and normal brain tissue is difficult to identify with a conventional surgical microscope. Intraoperative visualization of diffusely invasive tumor lesions with a surgical microscope is also a major challenge [17].
Several kinds of intraoperative image-guided surgical techniques for glioma have been developed, e.g., neuronavigation, ultrasonography, MRI, and fluorescence-guided surgery [18-22]. 5-ALA is the most studied and the most used of the various fluorescent tumor markers for fluorescence-guided surgery. It localizes the malignant glioma more precisely for its surgical removal [23, 24]. 5-ALA is a metabolite in the heme biosynthesis pathway. Exogenously administered 5-ALA is metabolized to fluorescent PpIX via the heme biosynthesis pathway in active proliferating tissues. After administration of 5-ALA, a malignant glioma produces and accumulates an excessive amount of intracellular PpIX compared to the surrounding normal brain parenchyma [25]. The PpIX within the tumor emits red fluorescence under violet-blue light [26].
Optimal equipment for the intraoperative visualization of porphyrin fluorescence is essential for fluorescence-guided surgical removal of a brain tumor. Some existing optical devices are the surgical microscope, endoscope, exoscope, and surgical loupe system. The purpose of this review is to investigate the devices that can observe 5-ALA-induced fluorescence reported on to date to evaluate their indications, advantages, and disadvantages.
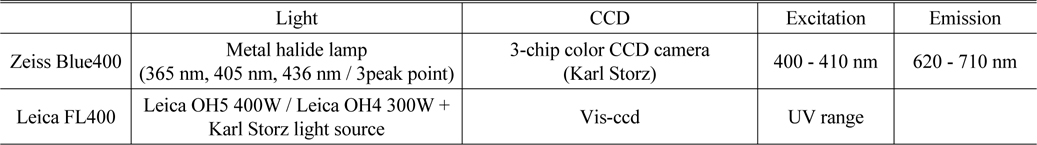
The specifications of the Zeiss BLUE 400 and the Leica FL400 are summarized in Table 1 [27, 28].
[TABLE 1.] Specification of Zeiss Blue400 and Leica FL400
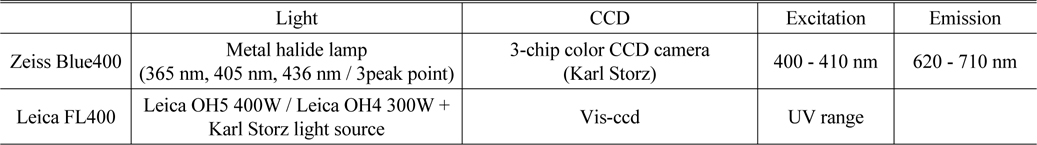
Specification of Zeiss Blue400 and Leica FL400
II. FLUORESCENCE IMAGING INSTRUMENTATION
2.1. Surgical Microscope System with 5-ALA
Porphyrin fluorescence can be visualized using a modified microscope and filtered light to help identify neoplastic tissue and perform maximal resection [29, 30]. The fluorescence imaging apparatus is mounted on the surgical microscope, the most basic piece of equipment in neurosurgery. A short-pass filter is placed in the excitation light path to filter out the proper wavelength for excitation of porphyrin to produce fluorescence, which is shorter than the fluorescence emission wavelength. A long-pass filter is placed in the light path of the observer to block out the excitation light and allow only red porphyrin-induced fluorescence to pass through. There are several kinds of commercially available surgical microscopes for 5-ALA fluorescence-guided surgery. For example, the BLUE 400TM module on the OPMI® Pentero® surgical microscope (Zeiss, German) contains special filters for excitation in the 400-410 nm wavelength range and display in the 620-710 nm wavelength range. Figure 1 shows the Leica FL400 module (Leica, German) on a surgical microscope. Figure 2 shows the surgery with 5-ALA using fluorescence microscopy system. And Fig. 3 shows the detect fluorescence image and original image by using fluorescence microscope.
The excitation spectrum and emission spectrum profiles overlap slightly so that both the normal brain cells, which re-emit a small fraction of the excitation light giving them a blue color, and the red PpIX-fluorescing glioma can be visualized simultaneously and, thus, the malignant tissue is seen in the context of its surrounding normal tissue without the need to switch to monochrome filters. The amount of spectral overlap is balanced to allow the passage of enough blue light to quench red autofluorescence without affecting the intensity of the true red fluorescence and thus obtain maximum image detail [31, 32].
At high-grade tumor margins, where recurrences are most likely to occur, photobleaching by PpIX may impair signal visualization [33]. Conversely, margins that have been resected because of the presence of a fluorescent signal occasionally have been shown to lack any histologic evidence of tumor cells. This suggests that there are general inflammatory or reactive changes contributing to the accumulation of the fluorescent marker. Initial reports on intraoperative quantification of fluorescence probes have shown improved specificity of tumor detection, but further refinement of the techniques involved is needed [34].
Most studies in 5-ALA-guided neurosurgery have been performed using fluorescence microscopy [35-38].
In 1998, Stummer
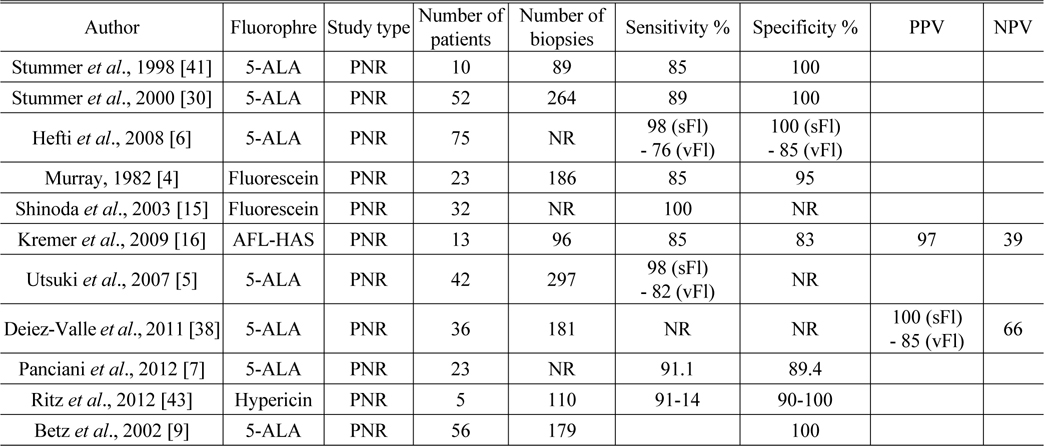
[TABLE 2.] Sensitivity, specificity, PPV, and NPV for fluorophores
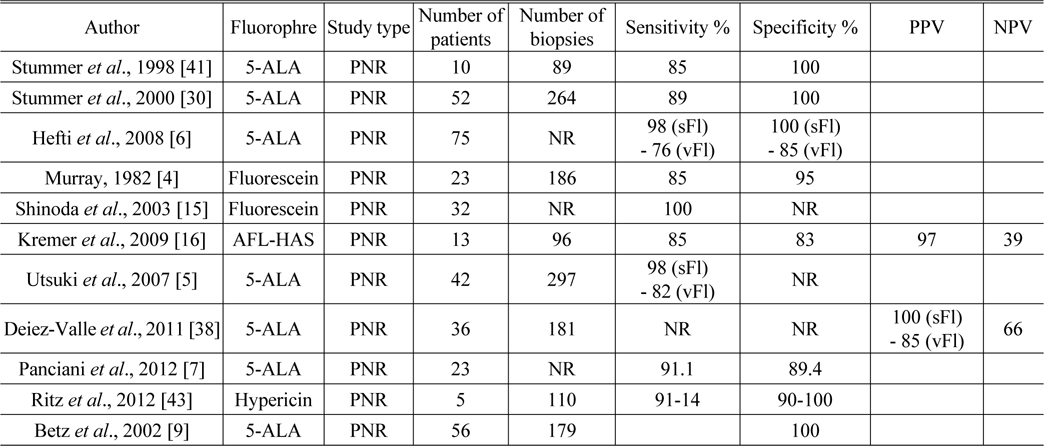
Sensitivity, specificity, PPV, and NPV for fluorophores
The ALA-Glioma study group conducted a randomized controlled multicenter phase III trial and published the results in 2006 [39]. A total of 322 patients with suspected malignant glioma were enrolled in this trial and randomized to either conventional white-light microsurgery or 5-ALA fluorescence-guided resection. Complete resection was performed in 65% of the intervention arm compared with 36% in the control arm. There was a 29% overall reduction in the number of patients with residual tumor who underwent early MRI in the ALA group compared with the group that underwent standard white-light surgery. Progression-free survival (PFS) at 6 months was greater among the patients assigned to the ALA group than those who underwent white-light microsurgery. However, overall survival did not differ significantly between the two groups. Median survival was 15.2 months (95% [confidence interval] CI 12.9-17.5) in the intervention arm and 13.5 months (95% CI 12.0-14.7) in the control arm (HR [hazard ratio] 0.82, 95% CI 0.62-1.07). Median PFS was 5.1 months (95% CI 3.4-6.0) in the intervention arm and 3.6 months (95% CI 3.2-4.4 months) in the control arm. There was no clear evidence that this intervention improved overall survival. In addition, neurological deterioration appears to be more common after fluorescence-guided surgery in which 5-ALA is used.
There are some limitations with this technique with respect to subjective fluorescence interpretation, especially at the tumor margins. Although fluorescence intensity is high in the central part of the tumor mass, it might be relatively weak at the tumor margins. The distortion of the optical properties of spatially heterogeneous tissue leads to subjective interpretation of PpIX fluorescence and is a limitation of this technique as currently practiced. Both excitation light and fluorescence emission, as attenuated in tissue, make the detected fluorescence prone to subjectivity. This subjective fluorescence significantly decreases the correlation between the true level of fluorophore and the visualized fluorescence.
Photobleaching is the chemical degradation of a fluorophore resulting from exposure to light [40]. The risk of photobleaching of a fluorophore increases with the length of exposure to and intensity of light, and when its concentration and photochemical stability are low. The intensity of excess normal white light can easily be reduced to 25%. The photobleaching effect is relevant mainly at the tumor margins where PpIX concentrations are low [41].
Increasing the extent of resection risks encroaching upon healthy brain cells. Individual trial results have suggested that the use of 5-ALA increased PFS compared with standard surgery. However, it appears that neurological deterioration is more common after 5-ALA-induced fluorescence-guided surgery: 12.4% in the intervention arm and 11.6% in the control arm suffered significant neurological adverse events (AEs). Neurological deterioration may be more common soon after the use of 5-ALA; however, there does not appear to be any long-term morbidity.
The tumor mass might not be completely removed because the surgical microscope may limit the light that goes into the cavity, e.g., the subcortical parts such as the overhanging margins of a large spherical tumor might not be visualized. In addition, residual tumor tissue in deep-seated resection cavities might not be detected because of the exponential decline in excitation light intensity with increasing distance from the light source.
2.2. Neuroendoscopy with 5-ALA
An endoscopic procedure for which 5-ALA is administered is well established as a diagnostic tool for cancers such as bladder, oral, laryngeal, esophageal, bronchial tract, and peritoneal cancer [42-45].
5-ALA-induced fluorescence has been used in neurosurgery, but only in conjunction with a surgical microscope. However, microsurgical resection of a deep-seated malignant brain tumor is often not possible, so a combined diagnostic and therapeutic strategy is highly recommended. On the other hand, there are a few reports on the detection and resection of gliomas using an endoscope combined with a fluorescence-guided surgical system. Figure 4 shows the detect fluorescence image and original image by using a neuroendoscopy.
In 2007, Tamura
Ritz
More recently, Rapp
A neuroendoscopy capable of detecting fluorescence can detect intraoperative residual tumor tissue in real time without extensive time-consuming methods. Endoscopy-assisted fluorescence-guided resection can detect additional fluorescent tissue in a blind spot or tissue not visible to the conventional microscope.
Another interesting optical device for fluorescence-guided surgery for glioma resection is the exoscope. The exoscope consists of a tubular telescope connected to a camera and a high definition monitor. It obtains high-quality images with a wide field and a target distance of 200 mm. The wide range allows the exoscope to be set far from the surgical field. The instrument can be used with fluoroscopy, which is not possible with conventional neuroendoscopy. The exoscope also can be used to visualize deep lesions.
Figure 5 shows the exoscope system in neuronavigation-guided biopsy. And Fig. 6 shows the original image and fluorescence image by using an exoscope with 5-ALA.
The exoscope is lighter, less expensive, and easier to use in a teaching environment compared with a surgical microscope. However, the surgical microscope remains the better optical tool with respect to resolution, color fidelity, and stereoscopic vision.
Piquer
In 27 cases of high-grade gliomas (23 GBMs, 2 anaplastic astrocytomas, and 2 anaplastic oligodendrogliomas), gross total resection was achieved in 20 (79.3%). The authors observed progression of hemiparesis after surgery in three patients, with subsequent partial recovery in two and transient dysphasia in one. These results are very similar to those observed in a larger series. The authors suggested that the use of the exoscope in 5-ALA-induced fluorescence-guided surgery for brain tumors helps achieve a more radical resection of the lesion.
2.4. Surgical Loupe System with 5-ALA
Currently available fluorescence-guided surgical microscope systems use a xenon light source with a broad wavelength range (~405 nm) as the excitation light source. The excitation light, which must be attenuated, passes through a band-pass or a low-cut filter that allows the passage of light with a wavelength of ~635 nm.
PpIX, a metabolite of 5-ALA, emits fluorescence when it is excited by the semiconductor laser excitation light source of the surgical loupe system, which exhibits a sharp peak at 405 nm. An UV cutoff filter is mounted on an eyepiece lens of the surgical loupe.
The advantages of this system are the good-quality PpIX fluorescence obtained with a narrow-band excitation beam and no need for filter on-off manipulation. The surgical loupe system is small, easy to use, and inexpensive compared to the large, complicated surgical microscope. Moreover, surgeons can use this surgical loupe system under white light to perform ordinary surgical procedures. However, the surgical loupe system has some disadvantages such as inferior quality of the optical imaging and a limited monitoring system. Figure 7 shows a head-mounted microscope for use in maxillofacial surgery.
2.5. Optical Spectroscopy with Fluorescence Imaging
Optical spectroscopy is used to observe the interactions of electromagnetic radiation with matter that occur in the UV, VIS, near-infrared (NIR), and infrared (IR) spectral ranges. Light in the UV/VIS spectral region (<700 nm) can penetrate superficial tissue only [47]. However, NIR light (700-900 nm) can propagate through tissue several centimeters thick because tissue does not absorb NIR radiation well [48]. Because of these features, multispectral fluorescence imaging of tissue is performed [49]. By recording the full fluorescence emission spectrum in each image pixel obtained with optical spectroscopy, subtle changes in tissue are observed by noting changes in the microenvironment. Multispectral fluorescence imaging has been widely used for diverse clinical studies.
The wavelength characteristics in the UV/VIS spectral region have been utilized extensively for the detection of precancerous and cancer cells in the surface of various organs such as the colon, cervix, bronchus, lung, and brain. In addition, NIR spectroscopy is emerging as a promising diagnostic tool by detecting blood flow within thick tissues [50-52].
NIR light can penetrate tissue up to several centimeters thick, so it can be used for fluorescence imaging. NIR imaging has been used in small-animal imaging experiments and has recently been expanded to clinical use. Optical technology is relatively less expensive and more portable than the systems discussed above, and it uses non-ionizing radiation and stable molecular markers. Therefore, optical spectroscopy with fluorescence imaging is expected to contribute to continued developments in molecular observations and biomedicine [53].
In a recent study, Hopton
Spectroscopy is used to obtain information that is used for the detection of disease or the molecular structure of the human body.
Fluorescence spectroscopy has been used for fast and minimally invasive detection of cancers and precancerous cells in a variety of organs, including the cervix, colon, bronchus and oral mucosa [55-58]. Moreover, fluorescence spectroscopy not only provides information on the molecular composition of the skin, it is also used to detect numerous skin diseases such as vitiligo, atopic and psoriatic skin, and basal cell carcinoma [59, 60]. Recently, fluorescence spectroscopy was used to detect Alzheimer’s disease in the brain [61].
III. CONCLUSION AND DISCUSSION
Recently, surgical equipment combined with fluorescent imaging has been used in various surgical procedures, e.g., the surgical treatment of glioma is typical of this trend.
A poor prognosis, including physical disability, is associated with glioma resection, depending on the site of the lesion. Use of 5-ALA in the fluorescence imaging detection system helps minimize the resection of the glioma, which will have a significant effect on the conservation of brain function. This advantage can increase the skills of the surgeon and be an immediate benefit to the patient. The system provides visual information to the surgeon, which allows an immediate response based on the provided information. It would be helpful if the system is improved by adding a fluorescence detection function involving ICG to allow identification of important blood vessels during brain surgery.
Fluorescence-guided surgery has some limitations. The fluorescence of a 5-ALA detection system is known to undergo photobleaching; it decays about 36% after 25 min in UV light and after 87 min in white light [62]. In addition, the system needs UV light with a wavelength ~405 nm to observe fluorescence induced by 5-ALA. However, the UV light source is a problem for the patient and the surgeon because UV light can directly or indirectly cause cataracts, macular degeneration, and amblyopia.
The system itself can lead to problems with the surgical procedure. All light sources except the UV light source must be turned off during surgery to be able to make observations. This decrease in light causes loss of information for the surgeon and the observational system.
The surgical loupe system resolves the disadvantage of UV light. This system is optimized for good-quality PpIX fluorescence imaging [63]. In addition, it is inexpensive and easy to use. However, it is difficult to obtain better-quality optical imaging and it has a limited monitoring system.
Fluorescence-guided surgery has many advantages but it also has some disadvantages. Therefore, numerous studies that will supplement the limitations discussed here are ongoing.
The exoscope system is one technology that can replace the microscope system. With this system, autofocus from a long distance is possible during surgery, so the surgeon does not need to adjust the focus with respect to depth. In addition, the exoscope system can detect tumors at different depths, and the loss of light can be reduced because the light path is shorter than that in a microscope.
A pen-type probe system enables researchers to observe the fluorescence image of tumors and blood vessels after surgery using a microscope, and it makes it possible to detect a residual tumor and critical blood vessels in real time. Less light is lost with the pen-type probe system because of the minimized distance between the surgeon and the object.
The spectroscope is used to detect the wavelength of a light source and thus can determine the wavelength of the fluorescence of a tumor during surgery. It does not require an additional imaging system to make observations, and it is easy to use.
Information needed by the surgeon and by the observation system that is lost due to the decrease in the amount of light can be compensated by image processing and registration. Surgeons can detect the fluorescence and the original image, which is provided via the image registration system, with improved clarity and brightness through image processing. Image registration technology assists in observing the position of the tumor and the blood vessels quickly and easily.
If the drawbacks of the systems presented here are resolved, the tumor resection rate can increase because microsignals generated by a low-grade tumor would be detected and the relapse rate would decrease. Furthermore, preservation of brain function by performing minimal resection is possible, thus enhancing the life of the patient after surgery by increasing the survival rate and reducing the incidence of disabling results.






![Surgical microscope fluorescence system of Leica FL400 [28].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f001.jpg)
![Surgery with 5-ALA using fluorescence surgical microscope fluorescence system of Leica FL400 [28].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f002.jpg)
![Detecting the fluorescence image of tumor and original image using fluorescence surgical microscope fluorescence system of Leica FL400 [28].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f003.jpg)
![White light image (A and C) and fluorescence image (B and D) with 5-ALA [64].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f004.jpg)
![Exoscope system in neuronavigation-guided biopsy [65].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f005.jpg)
![Detect the (A) original image and (B) fluorescence image of tumor using exosocope with 5-ALA [65].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f006.jpg)
![Dr. Roberto Pareschi, MD in the ENT Department of the Legano Hospital in Milan, Italy uses the headmounted microscope for the maxillofacial approach [35].](http://oak.go.kr/repository/journal/20776/E1OSAB_2016_v20n2_305_f007.jpg)