The determination of isotopic ratios of uranium (U) samples has applications in diverse fields including industrial use of uranium as fuel in nuclear power plants,1-2 nuclear safeguards,3-5 nuclear forensics,6 radiochronology studies,7-8 and even geochemistry and geochronology9-10 for disequlibrium studies. Accurate and precise isotopic ratio analysis of uranium and plutonium (Pu) in environmental safeguards samples is particularly important in monitoring undeclared nulcear activities.
Currently, there are a variety of techniques used for this determination, which vary remarkably in their sensitivity and accuracy. These techniques include counting-based nuclear spectroscopy, thermal ionization mass spectrometry (TIMS) and inductively coupled plasma mass sepectrometry (ICP-MS).11-14 The earliest U isotopic measurements were conducted by alpha-counting spectrometry.11 These methods consumed large amounts of sample material and required relatively long analysis times to obtain reliable analytical results. TIMS has long been the benchmark technique for the determination of long-lived radioisotopes such as U and Pu.12 However TIMS requires also relatively long measurement steps. ICP-MS is now the most frequently used inorganic mass spectrometric technique for concentration and isotopic ratio measurements.13-14 It is often a more effective tool than TIMS due to the increased ionization efficiencies for a given sample size.15 The types of ICP-MS instruments can be classified as quadrupole, high-resolution (HR), multi-collector (MC), and even time of flight (TOF) depending on the types of mass analyzer and detector, They vary in signal sensitivity, detection limits, precision, and dynamic ranges.16 Among them, the simultaneous measurement of different isotopes with a multicollector (MC) system is preferable for detecting ultra-trace levels of the analytes and reduces the uncertainty caused by fluctuations of the ion beam intensity over time.
There has been increasing interest in improving signals by modification of sample introduction systems due to an increase in the need to detect small amounts of U (sub-nanograms) in samples.17-19 One approach is to use a desolvating nebulizer system.17-18 It is mainly used to increase signal sensitivity and decrease the limit of detection for the hydride- and oxide-forming elements that are often present at very low concentrations. However, it also saves time by making it possible to simultaneously analyze other elements of interest. For these reasons, we combined the Aridus-II desolvation system with MC-ICP-MS (Neptune plus, Thermo Scientific, Germany) in our laboratory.
The Aridus-II consists of a low–flow PFA nebulizer (50-200 μL/min) and a heated PFA (perfluoroalkoxy alkanes) spray chamber with a PTFE (polytetrafluoroethylene) membrane desolvator unit. This combination provides enhanced analyte sensitivity by 4- to 10-fold compared to a normal spray chamber. In addition, solvent-based oxides and hydrides can be significantly reduced because sample solvent vapors are removed as they passing through an inert membrane toward the vent. Aridus-II is particularly advantageous for small-volume and highly corrosive samples. In addition, it can easily be interfaced with all current types of ICP-MS instruments. One disadvantage of the desolvating nebulizer system is a memory effect due to the relatively long nebulizer length compared to normal chambers. However, this memory effect can be minimized by cleaning it with an appropriate cleaning solution.
Signal stability plays an important role in isotopic ratio analysis and it is strongly affected by the sample flow rate. In many of cases, colloids in the sample solution and the formation of precipitates or an oxide layer on the inside of the nebulizer can hinder signal stability during mass analysis by providing irregular flow rates and even blocking the flow. It could be a fatal flaw when quantifying and estimating isotopic ratio with limited sample volumes. One way to avoid this problem may be to use a peristaltic pump in the sample introduction system to maintain a constant flow rate.20 Although this can lead to the signal pulsation corresponding to the flow pulses which arise as each individual roller of the pump squeezes the pump tubing,21 this is minimal when using a finely controllable peristaltic pump. In addition, simultaneous measurements using MC-ICP-MS could also minimize signal fluctuations caused by pulsation. For this reason, we combined a lowflow rate peristaltic pump with the Aridus-II system in the sample introduction system.
Here, we describe this system, an advanced technique that improves signal sensitivity, reduces formations of uranium hydride and improves signal sensitivity.
Isotopic ratio analyses of uranium solutions were performed using the MC-ICP-MS system (Neptune Plus, Thermo Scientific, Germany) with nine Faraday collectors and five ion counters.
Two different types of sample-introduction systems were compared: the quartz SSI cyclonic/scott dual spray chamber and the Aridus-II desolvation nebulizer system. Both were connected with a PFA nebulizer with a nominal uptake rate of 100 μL/min. A desolvation nebulizer system, consisting of a membrane desolvator and a spray chamber coupled with a PFA nebulizer (100 μL/min), was attached to the MC-ICP-MS to improve signal sensitivity and to reduce the formation of uranium hydride.
The low-flow rate peristaltic pump (BT-100-2J, Baoding Longer Precision Pump Co., China) was used to reduce long-term signal fluctuations in the uranium signal. The pump provides flow rates from 0.0002 to 380 mL/min by changing the speed from 0.1 to 100 rpm. The speed can be adjusted manually or automatically through an external control.
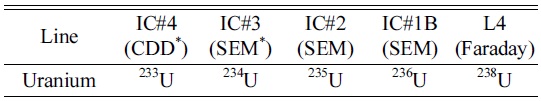
To monitor signal enhancement by Aridus-II, the ion counter (IC#1) was used with 5 pg/mL of U005 solution. To estimate of UH/U, the faraday cup (L4) and ion counter (IC#1C) were used for 238U and 238UH, respectively. The cup configurations of the measurements are shown in Table 2.
>
Chemical reagents and standards
Ultra-high pure grade nitric acid was purchased from Seastar Chemicals Inc. (67-70 wt.%, all metal impurities ≤ 10 pg/mL) All labwares were thoroughly cleaned with ultra-high pure bases (tetramethylammonium hydroxide, 10 wt.%, 1 M nitric acid) and Milli-Q water prior to use. We used 3% nitric acid as a working solution for MC-ICP-MS measurements. 5 pg/mL of U005 was used for signal-enhancement studies and 100 pg/mL and 500 pg/mL U005 were used to estimate the formation ratio of UH/U. CRM112A, U030 and U005 were used for isotopic ratio analysis of uranium. CRM112A, U030 and U005 were purchased from National Institute of Standards and Technology (NIST).
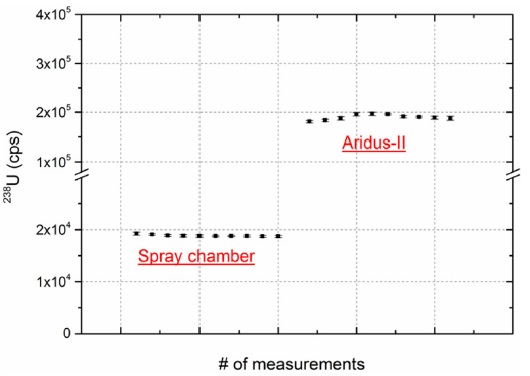
To investigate the effects of signal enhancement using the desolvating system, we compared the signal intensity of 238U with an ion counter (IC#1) using U005 standard solution containing 5 pg/mL of uranium between a normal spray chamber (Stable Sample Introduction (SSI) quartz dual cyclonic type) and the Aridus-II system. Because signal intensity is affected by the size of the PFA nebulizer (sample uptake rate) and skimmer cone type, we fixed the nebulizer with a nominal uptake rate of 100 μL/min (measured value was ~ 94 μL/min) and the same skimmer cone type (X type) in both systems. As shown in Figure 1, the overall sensitivity enhancement of 238U using the Aridus-II was approximately 11-fold because heated PFA spray chamber of Aridus-II system provides high sample transport efficiency by removing solvent molecules and PTFE membrane desolvator reduces solvent-based interferences such as oxides and hydrides compared to a normal spray chamber. The precision was 1.1% and 1.4% for the spray chamber and Aridus-II, respectively, indicating that both provide highly precise analytical results.
Quantifications and isotopic ratio analyses of uranium using ICP-MS can be complicated by the formation of polyatomic ions such as UH+ and UO+. Particularly, the formation of UH significantly interferes with isotopic ratio analysis of uranium in the presence of various other elements.22 For example, 233UH and 234UH overlap with 234U and 235U, respectively. 232ThH and 237NpH are also isobaric species of 233U and 238U, respectively. These hydrides are mainly generated by the injection of water vapor from a standard nebulizer and spray chamber setup. The PTFE membrane desolvator of the Aridus-II system can significantly reduce hydride levels. As shown in Table 1, the formation ratios of 238UH/238U obtained with a normal spray chamber were 3.34×10-5 and 3.16×10-5 in 100 pg/mL and 500 pg/mL of U005 standard, while those of 238UH/238U obtained with Aridus-II were 1.89×10-6 and 1.92×10-6 in 100 pg/mL and 500 pg/mL U005 standard. These values were the average obtained from a total of 10 measurements with the same U005 standard solution for 100 pg/mL and 500 pg/mL, respectively. For each measurement, peak intensities were measured 30 times and the average peak intensity with uncertainty of uranium isotopes was obtained. The formation ratios of 238UH/238U were significantly improved by 16- to 17-fold using Aridus-II. Generally, bulk analyses for nuclear safeguards sample target both uranium and plutonium. Therefore, it is important to reduce the 238UH/238U ratio because 238UH can interfere with 239Pu during such bulk analyses.
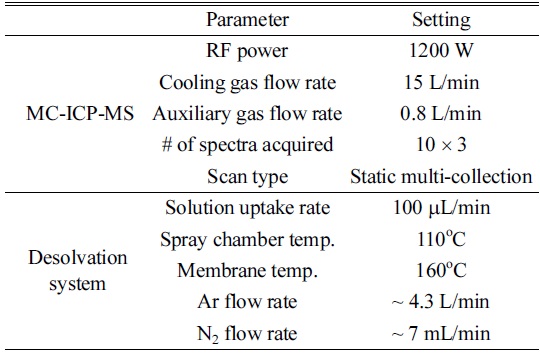
[Table 1.] Optimized operating conditions of MC-ICP-MS and a desolvation system
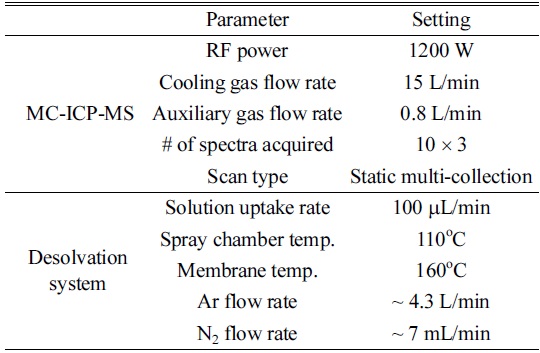
Optimized operating conditions of MC-ICP-MS and a desolvation system
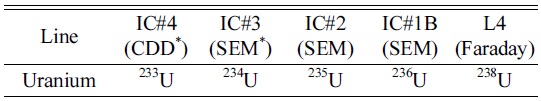
Cup configurations used for isotopic ratio measurements of U isotopes (simultaneous method)

238UH/238U signal ratios and their improvement when using the Aridus-II system compared to a normal spray chamber.
>
Optimization of signal stability
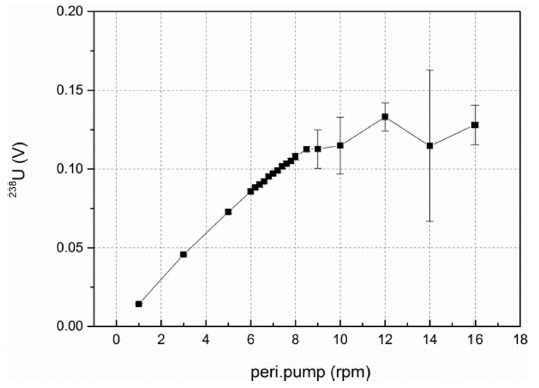
Next, we investigated changes in signal intensities and percisions depending on the speed of the peristaltic pump using the 100 pg/mL U030 standard. As shown in Figure 2, the signal intensities of 238U almost linearly increased as revolutions per minute (rpm) of the pump increased up to 8.5 rpm. However, the intensities were fluctuated at the high speeds (9 to 16 rpm). On the other hand, the RSD values of each data point (indicated as an error bar in Figure 2) were maintained within 2% up to 8.5 rpm. Hence, the optimal working range of the peristaltic pump is 6-8.5 rpm.
>
Isotopic ratios measurements
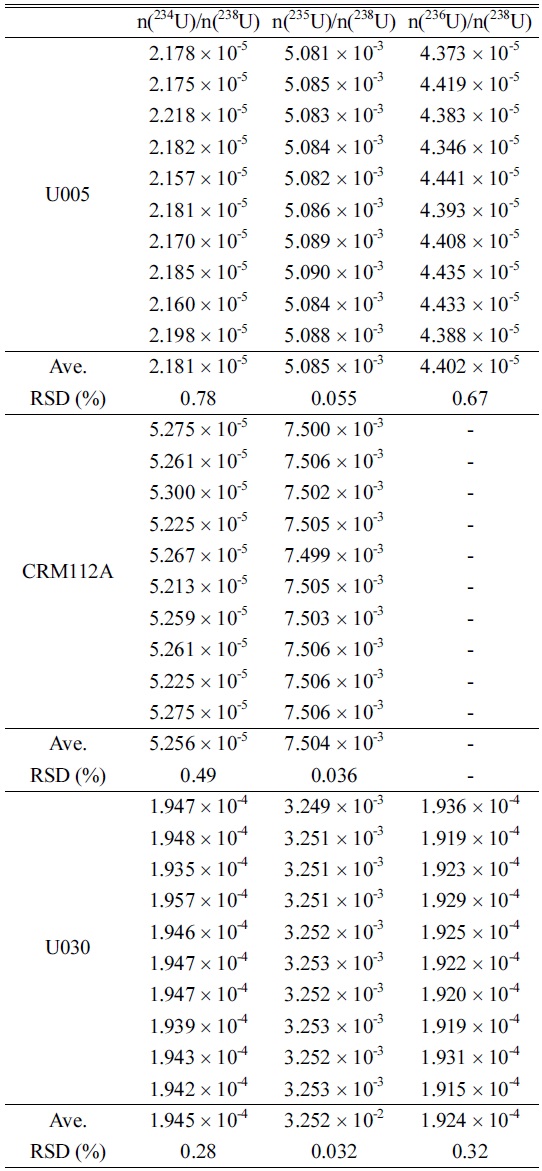
Generally, it is very important to estimate minor isotopes ratios (234U/238U, 236U/238U) as well as major isotope ratio (235U/238U) of uranium sample for nuclear safegards purposes. Therefore, we investigated the measurement precisions for isotopic ratios of several uranium standards such as U005, U030 and CRM112A (100 pg/mL), which have different isotopic ratios for 234U/238U, 235U/238U and 236U/238U. Similar to the estimation of 238UH/238U, the isotopic ratios of 234U/238U, 235U/238U and 236U/238U were obtained from the average values of repeated measurements (10 times). For each measurement, peak intensities were measured 30 times and the average peak intensity with uncertainty of uranium isotopes was obtained. The results are summarized in Table 4. The RSDs of 235U/238U were within 0.05% and those of the minor isotopes were within 1% for all standard samples at the 95% confidence interval. On the other hand, the minor isotopic ratios of all uranium standards at 100 pg/mL level were not able to be evaluated due to low signal intensities of 234U and 236U and the isotopic ratio of 235U/238U could be only estimated with relatively high RSD values (1.2%) when using a normal chamber without the Aridus-II and a peristaltic pump.
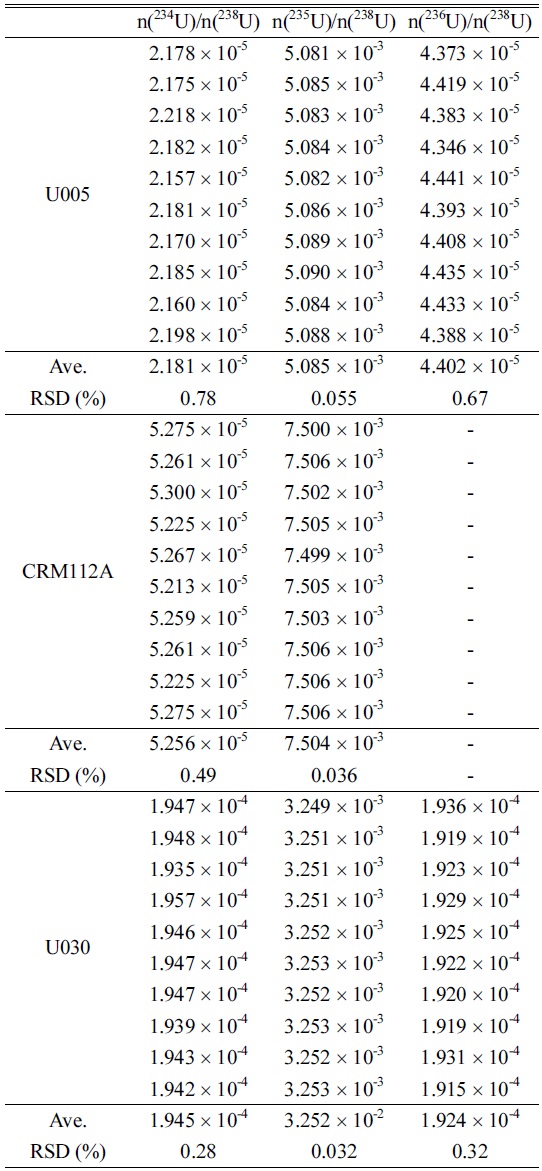
Isotopic ratios and RSDs of n(234U)/n(238U), n(235U)/n(238U), and n(236U)/n(238U) for the 100 pg/mL U005, CRM112A and U030 standards (No mass bias correction applied).
By optimizing the Aridus-II attached to the sample introduction system of the MC-ICP-MS, the signal sensitivity was enhanced approximately 11-fold and the formation ratios of 238UH/238U were reduced by 16- to 17-fold compared to the normal spray chamber. In addition, we confirmed that the use of a peristaltic pump combined with Aridus-II improved the long-term signal stability. The pump provided sufficient signal intensity and precision at the 6-8.5 rpm setting and the measurement precisions of isotopic ratio analyses using the pump and Aridus-II system together were within 0.05% for 235U/238U and were within 1% for 234U/238U and 236U/238U. Based on these analytical results, we believe that the experimental methodology described herein would be useful for quantifying and determining the isotopic ratios of other interesting elements at ultratrace levels.