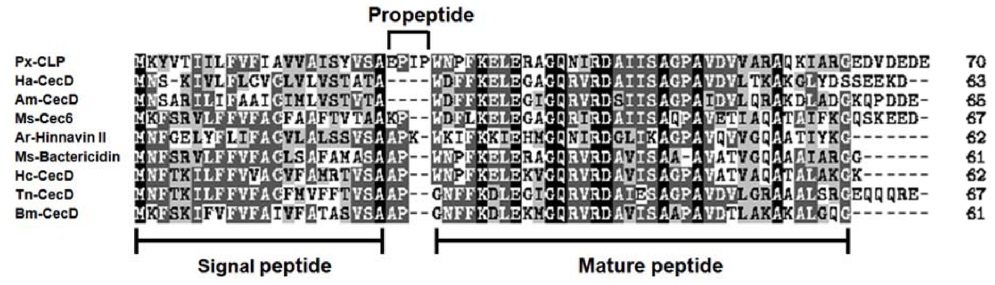
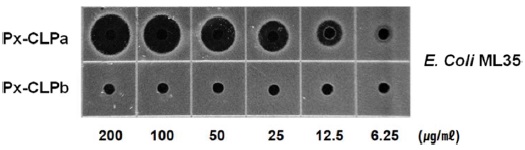
A new cecropin-like antimicrobial peptide (Px-CLP) gene was isolated from the immunechallenged larvae of the swallowtail butterfly, Papilio xuthus, by employing annealing control primer (ACP)-based GeneFishing PCR. The full-length cDNA of Px-CLP is 310 nucleotides encoding a 70 amino acid precursor that contains a putative 22-residue signal peptide, a 4-residue propeptide, a presumed 37-residue mature peptide, and an uncommon 7-residue acidic pro-region at the C-terminus. The deduced amino acid sequence of Px-CLP showed significant identities with other Lepidopteran cecropin D type peptides. RT-PCR revealed that the Px-CLP transcript was detected at significant level after injection with bacterial lipopolysaccharide (LPS). The peptides with or without C-terminal acidic sequence region were synthesized on-solid phage and submitted to antibacterial activity assay. The synthetic 37-mer peptide (Px-CLPa), which removed C-terminal acidic sequence region, was showed exclusively antibacterial activity against E. coli ML35; meanwhile, a 44-mer peptide (Px-CLPb) with C-terminal acidic peptide region was not active. This result suggests that Px-CLP is produced as a larger precursor containing a C-terminal pro-region that is subsequently removed by C-terminal modification.
Insect antimicrobial peptides (AMPs) accomplishes important role as key factors in systemic immune response against invading pathogens such as bacteria, fungi, and viruses (Hoffman
Cecropin, which is a well-studied antimicrobial peptide in lepidopteran insect immunity, was initially isolated from bacterially challenged
Previously, a 37-residue cecropin-like peptide named papiliocin was isolated from the bacteria-immunized larvae of the swallowtail butterfly
The swallowtail butterfly
>
RNA isolation and cDNA synthesis for annealing control primer (ACP) system
Total RNA were extracted from whole larvae at 12 h postinjection or untreated larvae using Trizol reagent (Invitrogen, CA) and then treated for 15 min with DNase I at 37 ℃ to remove any residual genomic DNA. TotalRNA were used for the synthesis of first-strand cDNA by reverse transcriptase. Reverse transcription was performed for 1.5 h at 42 ℃ in a final reaction volume of 20 μL containing 3 μg purified total RNA, 4 μL of 5x reaction buffer (Promega, USA), 5 μL of dNTPs (each 2 mM), 2 μL of 10 μM cDNA synthesis primer dT-ACP1, 0.5 μL of RNasin RNase Inhibitor (40 U/ μL; Promega, USA), and 1 μL of M-MLV reverse transcriptase (200 U/ μL; Promega, USA). First-strand cDNA samples were diluted by the addition of 80 μL of ultra-purified water.
>
Screening of differentially expressed genes (DEGs)
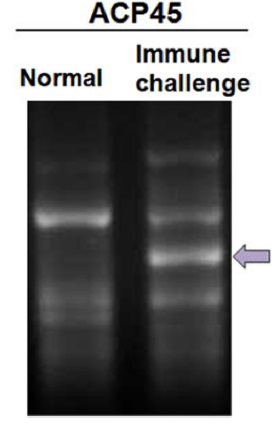
For screening DEGs in immune-challenged
>
Reverse transcription PCR (RT-PCR)
Total RNA were extracted from whole larvae 0 h, 12 h and 24 h post injection using Trizol reagent (Invitrogen, Carlsbad, CA) and then treated for 15 min with DNase I. The extracted total RNA samples (1 μg per sample) were reversely transcribed to cDNA using oligo (dT) primer and SuperScript III reverse transcriptase (Invitrogen, CA). PCR amplifications were performed with mixture containing cDNA, a pair of specific primers (5’-CGTCACAATCATCCTGTTCGT-3’ and 5-CATCTTCGTCTACATCCTCTC-3’) and taq polymerase mixture under the following conditions: 94 ℃ for 30 s, 62 ℃ for 45 s, and 72 ℃ for 1 min for 25 cycles with final extension at 72 ℃ for 10 min. The amplified PCR products were electrophoresed through 1 % agarose gel.
The peptides were synthesized by solid-phase synthesis method using Fmoc (9-fluorenyl-methoxycarbonyl) chemistry at the peptide synthesis facility, AnyGen Co. (Gwangju, Korea). The synthetic peptides were purified using reversed-phase high-pressure liquid chromatography (RP-HPLC) on a Waters 15-μm Delta Pak C18 column. Matrix-assisted laser desorption/ionization time-off-flight mass spectrometry (MALDI-TOF-MS) analysis was used to measure the molecular mass of synthetic peptide.
>
Antibacterial activity assay
The antibacterial activity of synthetic peptides were examined against Gram-negative
>
cDNA cloning and sequencing of Papilio xuthus cecropin-like peptide (Px-CLP)
In order to isolated immune-related genes from
>
Expression analysis of Px-CLP gene
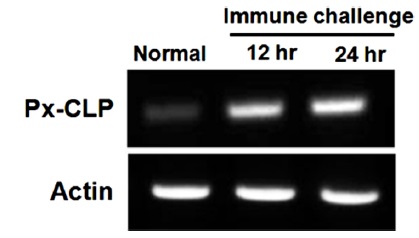
To confirm the expression of Px-CLP gene at transcriptional level, reverse transcription PCR (RT-PCR) analysis was performed using total RNA prepared form whole larvae at different time-points after LPS injection (Fig. 4). The result showed that no signal was detected when the larvae were not immunized, but transcript abundance increased significantly after immunization. The transcript of Px-CLP gene peaked at 12 h to 24 h after LPS injection. This result indicated that the expression of Px-CLP gene was rapidly induced after challenge. In larvae of
>
Antibacterial activity of synthetic Px-CLP
The amino acid sequence alignment showed that the precursor of Px-CLP contains an acidic pro-region (EDVDEDE) at C-terminus (Fig. 3), which may interact with the basic mature region. This acidic pro-region is also reported to be present in the tunicate cecropin type antimicrobial peptide styelin (Zhao
In conclusion, we cloned a novel member of cecropin-like antibacterial peptide (Px-CLP) gene from the immune-challenged swallowtail butterfly,