To detect pheromones and plant volatiles in the natural environment, insects have highly developed sensory organs and change their response on the basis of prior experience to eventually increase the chance of survival as far as possible. These odor cues play important roles in forging, habitation, mating, and ovipisition. In this regard, the olfactory system of insect need constantly adapt to environments due to the continuously changing environment. Thus, plasticity of sensory responses is required. The olfactory plasticity is a general phenomena induced by experienced odors (Claudianos
The effects of odor experiences on sensory systems demonstrate in a large variable time scale ranging from milliseconds to weeks, suggesting different temporal mechanisms of adaptation. It is known that odor response of animals declines with odor stimulation prolonged (Getchell and Shepherd, 1978; Firestein
Another exposure-induced adaptation is cross adaptation, which may extend to the other compounds. The widely accepted explanation of cross-adaptation is that animals have multiple pathways for passing stimulus information to the central neuronal system (CNS), and each pathway has a different molecular receptive range. The cross adaptation was widely found in insects and other animals. For instance, exposing the antenna of European corn borer moth,
Here, to further understand the self- and cross adaptation in insects, we for the first investigated the EAG responses of
Male
The stimulus chemicals, (9Z, 11E) - tetradecadienyl acetate (Z9,E11-14:OAc), (9Z, 12E) - tetradecadienyl acetate (Z9,E12-14:OAc), 9Z-tetradecen-1-ol (Z9-14:OH) and (Z)-7-dodecenyl acetate (Z7-12:OAc) were synthesized by NewCon Inc., Ningbo, Zhejiang province, China. All compounds were confirmed to be 92 - 96% chemically pure by GC analysis. Two different binary blends of Z9,E11-14:OAc and Z9,E12-14:OAc, which are main components in
>
Electroantennogram (EAG) recordings
Recordings of electrical activity of whole-antennae in response to volatile stimuli were made according to standard techniques. A male moth was stabilized in a 1-mL plastic pipette with a cut tip to allow only the antennae to protrude through the opening. The tip of one of the antenna was cut, and a recording electrode filled with Beadle-Ephrussi Ringer was placed in contact with the cut surface of the antenna and another at the base of the antenna. An Ag/AgCl wire serving as a ground electrode was inserted into the insect’s abdomen. The antenna was continuously flushed with moistened air stream, which was purified by a charcoal filter in a glass tube (8 mm i.d.). The outlet of the tube was about 20 mm from the antenna. The stimulus was injected into the air stream through a Pasteur glass tube 15 cm upstream from the antenna. The stimulation was delivered at a flow rate of 5 mL/s in 0.5-s puffs using a stimulation device (Syntech, The Netherlands). The signal was amplified using a high impedance amplifier, as well as stored and analyzed with the EAG2000 software.
The EAG calibration curve started with the blank (paraffin oil), followed by 0.02 ng, 0.2 ng, 2 ng, 20 ng, and 200 ng A-blend and B-blend as a positive control. Then two stimulationtrain protocols were used with 0.02 ng, 0.2 ng, 2 ng, 20 ng, and 200 ng Z9,E11-14:OAc or Z9,E12-14:OAc, respectively: either 10 cycles of 200 ms on/off, or 10 cycles of 1 s on/off. Each treatment replicates six times. The comparison among treatments was performed on transformed data , where x is the average value of ratios of first and last EAG amplitude of Z9,E11-14:OAc or Z9,E12-14:OAc; y is the average value of EAG amplitude of paraffin oil.
>
Self-adaptation and cross adaptation
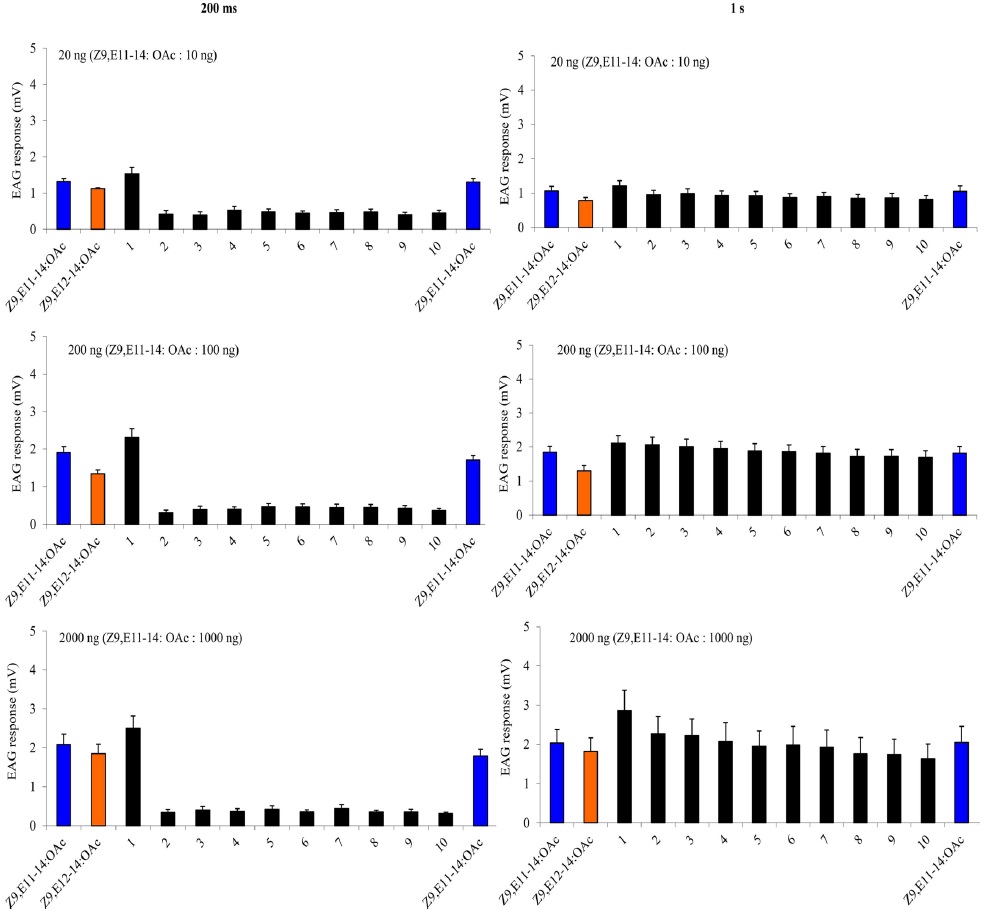
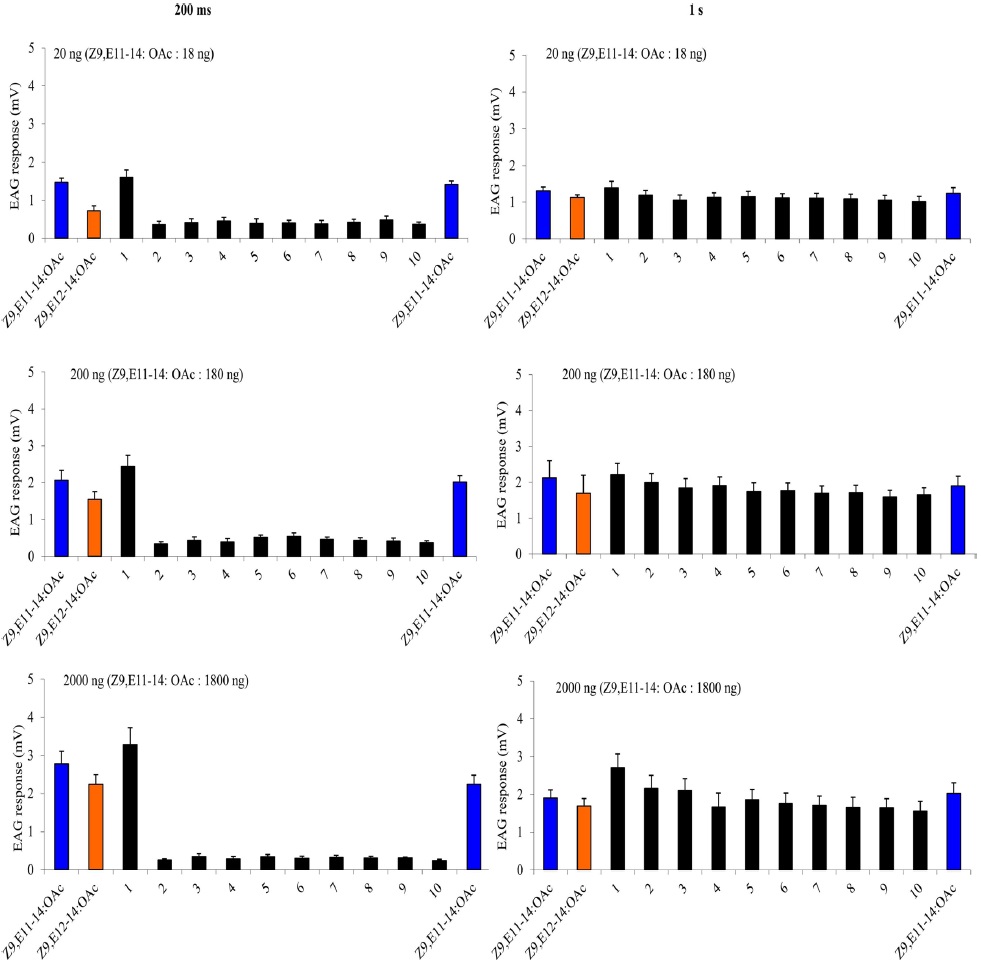
A charcoal-filtered, humidified air stream (1 L·min−1) was directed continuously over the antenna via a glass tube (20 cm length × 6 mm i.d.), positioned ~2 cm from the antenna. The selfadaptation EAG consisted two parts. The first one started with a either a 1-s or a 200-ms (depending on the protocol) puff of 20 ng, 200 ng, 2000 ng of Z9E11-14:OAc, followed 1 min later by a puff of Z9E12-14:OAc with the same dosage. Then one min later, two stimulation-train protocols as described above were used with A-blend and B-blend with the same dosages, finally followed by a single stimulation with Z9E11-14:OAc or Z9E12-14:OAc. The second self-adaptation EAG started 2000 ng either Z9E11-14:OAc, Z9E12-14:OAc, Z9-14:OH or Z7-12:OAc, and after one min, the stimulation-train of the same chemical with 10 cycles of 200 ms on/off was conducted. Finally, a single stimulation with the same chemical followed immediately.
The cross adaptation EAG started with 2000 ng either Z9E11-14:OAc, Z9E12-14:OAc, Z9-14:OH or Z7-12:OAc. After one minute, the stimulation-train of one of the other three chemicals with 10 cycles of 200 ms on, 200 ms off was conducted. Finally, a single stimulation with the same chemical to the first one followed immediately. Each antenna was considered a replicate of an adaptation treatment. The order of the puff treatments for each replicate was randomized among antennae. Each treatment replicates six times. To estimate the amount of adaptation the mean of the three puffs of a given treatment was divided by the mean of the three puffs of paraffin oil. This normalized EAG response after adaptation was divided by the equivalent response before adaptation (= adaptation index). The data were transformed [log(x + 1)] and analyzed with ANOVA followed by a least square analysis (significance level:
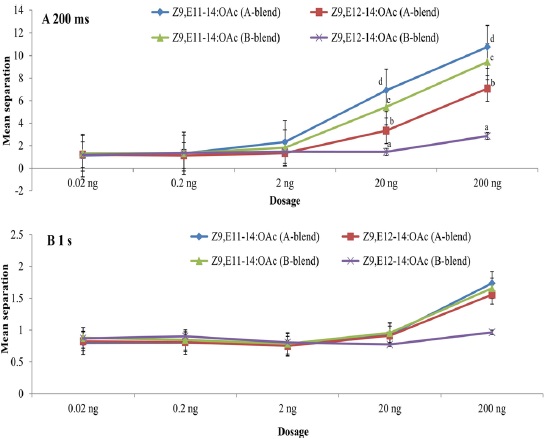
Using the pulse train with 200 ms, both Z9,E11-14:OAc and Z9,E12-14:OAc had significant effects on EAG peak amplitude at dosages of 20 ng and 200 ng whatever A- or B-blend was used as the positive control (Fig. 1A). Nevertheless, no significant effect was found when using the pulse train with 1 s even at the highest dosage, though there are somewhat differences among the compounds with positive controls (Fig. 1B). The highest mean separations of all treatments were consistently found at the highest dosage 200 ng, followed by 20 ng. The interaction of all treatments was not significant at the low dosages of 0.02 ng, 0.2 ng, and 2 ng because of their much lower responses (Fig. 1). The highest EAG responses at dosages of 20 ng and 200 ng were to Z9,E11-14:OAc with A-blend, followed by Z9,E11-14:OAc with B-blend, Z9,E12-14:OAc with A-blend, and Z9,E12-14:OAc with B-blend as positive control (Fig. 1).
>
The EAG adaptation of S. litura main pheromone compound to A- and B-blend
The challenge of
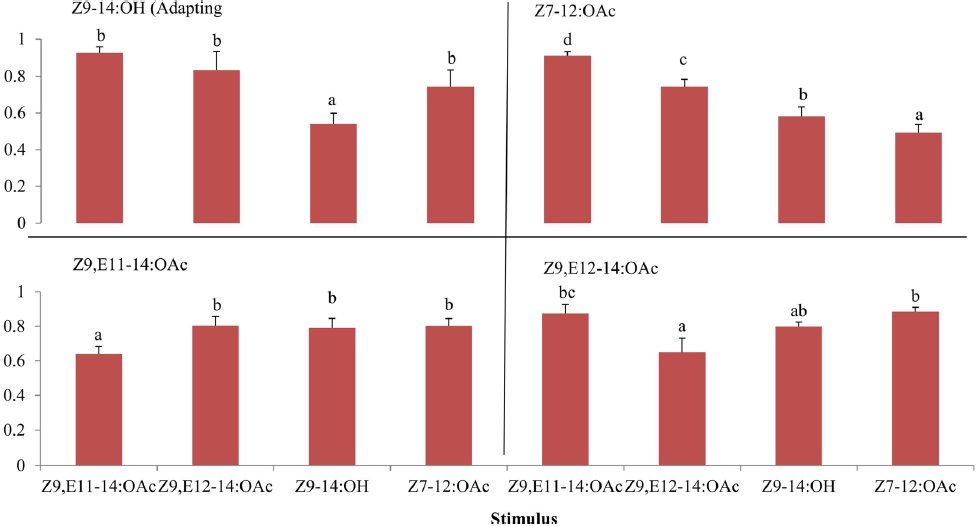
The effects of adaptation of
Adaptation in the sensory organ is an early step in the processing of sensory information, which together with central nervous integration enables the organism to cope with complex natural stimuli. A continuous stimulation may cause desensitization in odor responses, termed as sensory adaptation. The sensory adaptation has been widely reported in insects, exhibiting a reduction of responses in antennal sensory neuron to pheromone and central nervous system (Gemeno
Pheromone orientation in moths is an excellent example for studying adaptation. We stated the question how blend quality processing in dynamic plumes affects the antennal responses. Z9,E11-14:OAc and Z9,E112-14:OAc are main components in
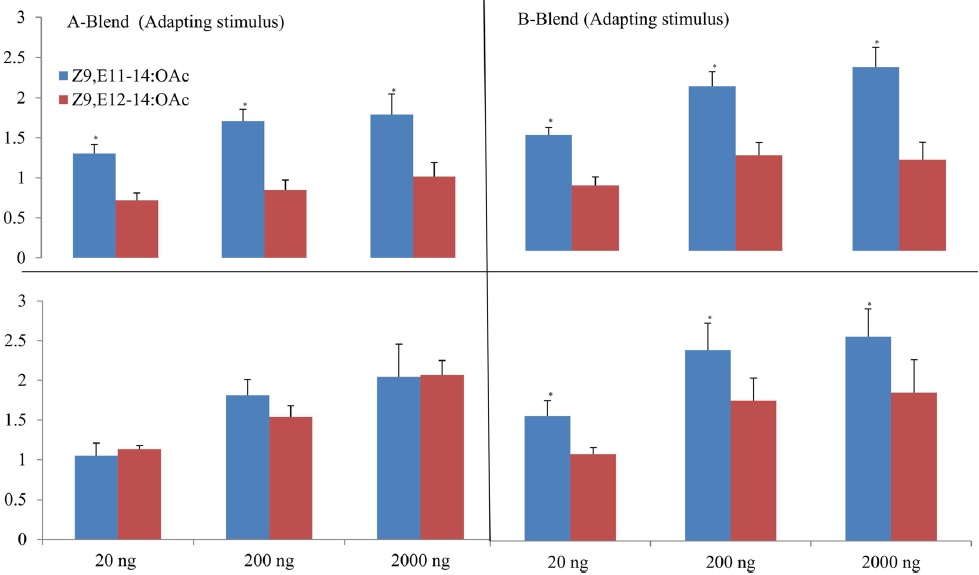
The cross-adaptation of Z9,E11-14:OAc and Z9,E112-14:OAc to A- and B-blends with different dosages showed that Z9,E11-14:OAc did not adapted to any a blend whereas Z9,E112-14:OAc exhibited adaptations to A- or B-blend in some cases, probably suggesting a different odor coding between the two components in the antennal periphery of
The results of cross adaptation EAG showed that a continuous stimulation of Z9,E11-14:OAc (df = 3, F = 6.81,
In current study,