Nano-electrospray (nano-ESI) was introduced by Wilm and Mann1 little more than 20 years ago, and it was named to reflect the flow rate and size of a droplets produced by emitters. While conventional electrospray typically uses nitrogen or argon to assist in droplet formation and operates at a flow-rate of 1-200 microliters per minute, nano-ESI produces a stable ion current at a flow rate of 10-100 nanoliters per minute. The flow rate of nano-ESI mainly depends on the inner diameter of the emitter tip and on the viscosity of the solution. It has been said that the efficiency of electrospray techniques to produce ions rose 500 times with invention of nano-ESI.2
The focus for the development of electrospray ion sources has always been on the emitter design, as an emitter’s geometry is the major factor influencing performance. One approach to increasing ionization efficiency is to minimize the size of the emitter tip. Whenthe diameter of a tip is reduced to a couple of micrometers, much smaller charged droplets are generated. The development of nano-electrospray (nano-ESI) extended the application of ESI making possible analysis of analytes obtainable only in small quantities, such as non-covalent complexes of proteins.
Generally, single-channel glass capillary emitters are used in nano-ESI experiments, and are commercially available. Dual-channel emitters, sometimes referred to as theta-shape capillaries however must be produced by researchers at the present. In order to achieve optimum geometrical parameters of the emitters, a good understanding of the manufacturing process is required. Electric contact to an emitter can be introduced by sputter deposition of a metal film, or by a metal wire inserted at the back of the emitter into the spraying solution.
Theta-shaped dual channel emitters were first described by Mark et al.3 The emitters were pulled from round borosilicate capillaries, which had a septum wall in the middle creating a so-called theta shape. Electrical contact was introduced in two ways - by hand painting of platinum paint and by sputtering of gold above a titanium adhesion layer. In this original study, hydrogen/deuterium exchange between vancomycin and deuterated vacomycin and interaction of vacomycin with diacetyl-l-lysyl-d-alanyl-d-alanine peptide (KAA) were described. It was shown that there is interaction between these two solutions and it was proposed that mixing occurred in a shared Taylor cone region.
Later theta-shaped emitters were used to effect changes in charge state distributions.4 Electrical contact was achieved by a platinum wire inserted into one or both channels at the rear end of the emitter. Mixtures of myoglobin and cytochrome c with acids and supercharging agents at different concentrations were sprayed with single and theta capillary emitters, and premixed solutions were compared with the short-time mixing processes. There were no difference found between mass spectra. During myoglobin-acetic acid experiments it was shown that myoglobin was partially denaturated and charge state distribution was changed, while mass spectra for premixed solution indicated only apomyoglobin. It was observed that charge state distributions shifted towards lower charge states for both cytochrome c and myoglobin.
Another study of myoglobin and cytochrome c with the use of theta shaped emitters was carried out to investigate folding and unfolding processes by altering pH of the solution.5 An approach to estimating time of interaction between two solutions based on the integrated law of a two-state folding reaction has been performed. A simplified mixing model based on Fick’s second law of diffusion was suggested for mixing in a droplet.6 It was stated that significant amount of mixing was occurring in droplets rather than in a Taylor Cone. To extend mixing times of two solutions with the use of theta shaped emitters, different voltages were applied to platinum electrodes to induce electro-osmotic flow from one channel to the other.7
The emitters have been produced from “theta-shaped” borosilicate capillaries (O.D. 1.2 mm, Hilgenberg borosilicate capillaries, # 1423021). The fabrication of electrospray emitters was performed on a laser-based micropipette puller. A glass tube was fixed between two holders and a laser beam shone across the middle of the glass tube. Application of heat melts the glass and after a certain time the force applied to a tube holders begins to pull apart the two halves of the glass tube creating two emitters. We used the P-2000 micropipette puller from Sutter Instrument Company. It has a carbon dioxide laser-based puller, which permits production of ESI emitters from a wide range of glass capillaries including quartz.
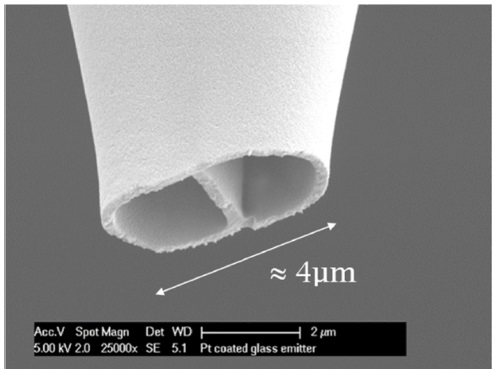
Dual-channel emitters pulled from the borosilicate theta-shape glass tubes had tips diameters of about 4 μm. Scanning electron microscopy (SEM) was used to examine the tips of the dual-channel emitters, shown in Figure 1.
Electrical contact was introduced either by deposition of a thin film of an appropriate metal onto the surface of emitters or by a platinum wire inserted at the back of the emitters. Usually noble metals are used for a metal coating in order to prevent sample contamination with metal ions. Noble metals are resistant to corrosion and oxidation in air, which allows storage of emitters for long times if required. Noble metals do not adhere well to the glass and, if they are coated without an adhesion layer, they can be easily removed from the glass by hands during handling.
Coating was performed in two steps:
During the metal deposition process, a septum wall in a dual-channel emitter was coated with metal as well the outer surface. A numerical simulation was performed using COMSOL Multiphysics to investigate how coating the septum changed the electric field around a dual-channel emitter.8 It was found that the strength of electric field around a dual-channel emitter had a magnitude about one and half times larger than for a single-channel emitter. This numerical simulation result was consistent with experimental results, as it was observed that a stable Taylor cone for dual-channel emitters was formed at a smaller electric potential compared to single-channel emitter (4.5 kV and 5 kV respectively for the capillary emitters with 1.2 mm outer diameter). Furthermore, the size of a Taylor cone in a dual-channel emitter was larger and had slightly different shape than a Taylor cone in a single-channel emitter.8
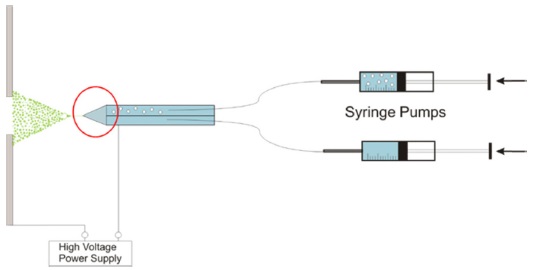
To investigate the fluid dynamics in a Taylor cone region a flow-visualisation experiment was performed for single- and dual-channel tubes with outer diameter 1.2 mm. Apparatus for the flow-visualization experiment is shown in Fig. 2.
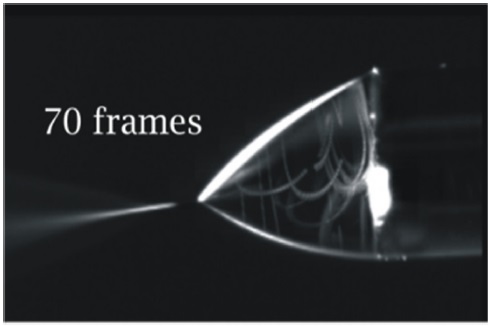
Polystyrene beads (Phosphorex Inc., #114) of three micrometres size have a density similar to water 1050 kg/m3. These small particles with density similar to the liquid solution were introduced at very low concentration (less than 0.04% volume) into one of the channels and the motion of particles was record with a high-speed camera. Fifty percent methanol in water was used as the liquid phase with density of 912 kg/m3. Two Cole-Palmer Instrument syringe pumps (74900 Series), holding Hamilton 100 microliters syringes were used for continuous solution injection and flow rate was set to 125 microliters per hour. The expectation was that the results of these experiments would give information on how electrospray was initiated and developed. Mixing processes were visualized the in the Taylor cone. Images were recorded with a high-speed camera at 1000 frames per second. A picture the fluid motion is shown on Figure 3. This picture was obtained by superposition of 70 frames.
As indicated in Fig. 3 particles were exploring the whole space in the Taylor cone region. These particles were moving in a helical or cork screw type of motion everywhere in a Taylor cone region. Similar flow patterns were obtained when particles were introduced into a single channel emitter, and in the results reported by Barrero et al.9 for polystyrene beads of 5-micrometer size were introduced to ethanol. Implementation of a similar experiment for nanoscale emitters is rather challenging, as particles should be less or around 1 nanometer.
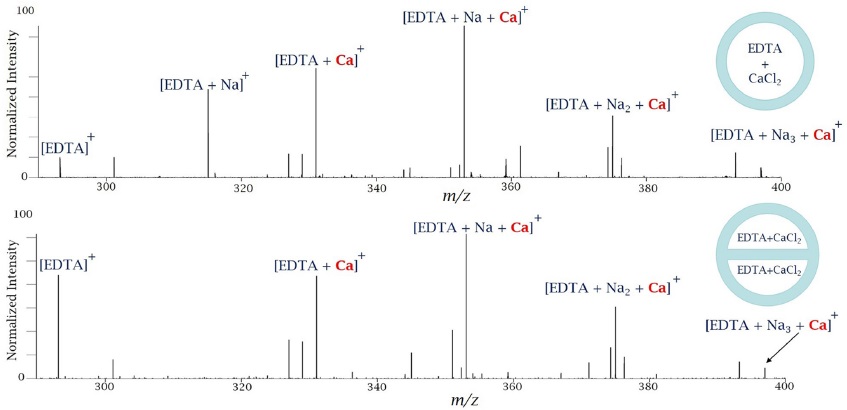
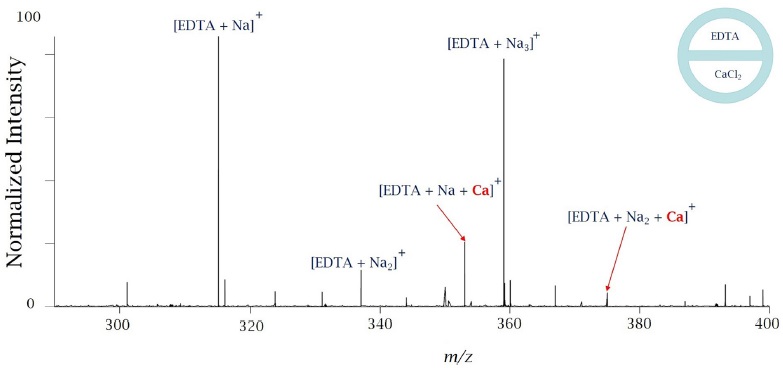
To investigate interactions of two different analytes in dual nano-electrospray a series of experiments with a simple system of metal-binding ligand ethylenediamine-tetraacetic acid (EDTA) and solutions of metal salts was performed. EDTA can bind up to six ions by losing up to four hydrogens. It prefers, however, to bind one or two metal ions. In the first set of experiments, metal-binding ligand EDTA at 10mM was injected in one of the channels and a solution of CaCl2 at the same concentration was injected into the second channel. Mass spectra (LTQ Fourier transform ion cyclotron resonance mass spectrometer (Thermo Finnigan))10,11 of EDTA premixed with calcium chloride in a single-channel emitter and the same solution loaded into both channels of a theta shape dual emitter are shown in Figure 4.
The mass spectra were very similar. There was less sodium when theta-shape emitters were used, which was reflected in a higher intensity of EDTA In the first set of a short time scale experiments, metal-binding ligand EDTA at 10 mM (pH=8) was injected in one of the channels and a solution of CaCl2 at the same concentration was injected into the second channel. The results of this experiment are shown in Figure 5.
There are strong peaks due to sodiated EDTA. There are no peaks due to calcium alone bound to EDTA, although there are peaks due to calcium bound together with sodium. This suggests that mixing is occurring in a very small volume and on a short time scale. As it was shown earlier, the fluid flow inside a Taylor Cone might be very rapid and it has a helical type of motion. Two different solutions may mix in a different volume fraction within a same Taylor Cone volume over time, and therefore equilibrium may not be reached during some reactions. Similar results were reported earlier for proteins by Mark et al.3 and later were confirmed by Mortensen et al.6
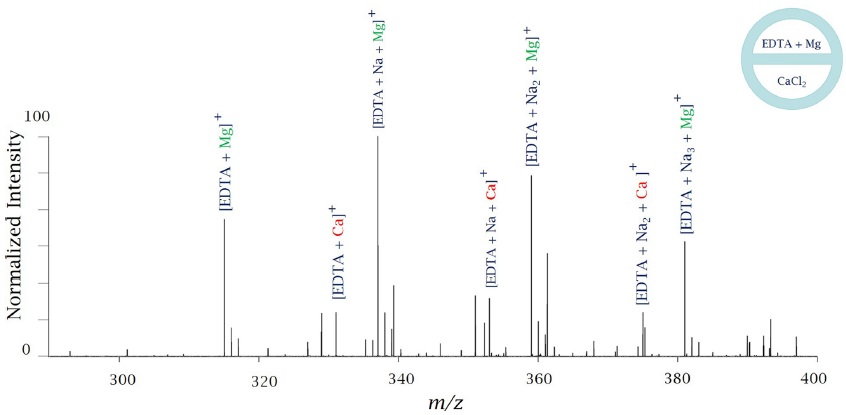
In a second set of experiments, EDTA mixed with magnesium sulphate at 10 mM (pH=8) was injected into one of the channels and calcium chloride at 10 mM (pH=8) was injected into the other channel. Magnesium ions have a lower affinity constant with EDTA, compared to calcium ions. In a solution of these two ions with EDTA, calcium ions would bind and replace magnesium ions. The aim was to observe an exchange process or replacement of magnesium with calcium. EDTA in a mixture with magnesium sulphate premixed a couple of hours before experiments was injected into one of the channels and calcium chloride was injected into second channel. As seen from the mass spectra (Figure 6), there is EDTA with both magnesium and calcium attached.
These results indicates partial replacement of magnesium ions with calcium occurred in theta-shaped dual channel emitters, and, as in case of EDTA and calcium, equilibrium was not reached.
The use of nano-electrospray emitters containing two separate channels has revealed the possibility to study interactions of two different solutions in a short timescale. One of the most important questions to be answered, however, is where does this interaction occurring. There are three ways of how two different solutions might interact:
Using borosilicate theta-shaped glass emitters coated with gold, it has been demonstrated that there was mixing in the Taylor cone region when