Sea anemones provide microhabitat, shelter, and trophic links for a variety of ectosymbiotic species, especially crustaceans (Bruce, 1976; Ross, 1983) and fishes (Dunn, 1981; Fautin and Allen, 1997; Arvedlund et al., 2006; Karplus, 2014). The gigantic sea anemone Stichodactyla gigantea (Forsskål, 1775) occurs throughout the Indian and western Pacific Oceans, typically inhabiting shallow areas with sandy substrates such as the margins of seagrass beds in reef lagoons (Dunn, 1981; Fautin and Allen, 1997). At least three species of decapod crustaceans, an unidentified sea cucumber, and eight species of fishes have been reported associating with S. gigantea (Dunn, 1981; Fransen, 1989; Fautin and Allen, 1997). However, much remains to be learned about the relationships between S. gigantea and its ectosymbionts. In this study we quantitatively describe the association of four species of decapod crustaceans, two sea cucumbers, and a cardinalfish with S. gigantea in Kosrae, Micronesia.
Rising to an elevation of 630 m above sea level, Kosrae (5°19′N, 162°59′E) represents the easternmost high island of the Caroline Islands of Micronesia in the tropical western Pacific Ocean. It is a 110-km2 volcanic island surrounded by fringing coral reefs and a few small satellite islands. The marine environment of Kosrae was described by Eldredge et al. (1979).
We searched for S. gigantea anemones in suitable shallow-water habitat at Walung, Tafunsak, and Lelu (Fig. 1) while wading, snorkeling, kayaking, and motorboating during 19-28 Jun 2013 and 20-27 Jun 2014. For each S. gigantea found we measured its size by measuring the maximum width (nearest cm) of its oral disc with a plastic ruler or by photographing the anemone beside an underwater writing slate of known length and later extrapolating the maximum width. We searched each S. gigantea for ectosymbionts and counted the number of individuals present. We attempted to photograph each species of ectosymbiont to confirm its identification by consulting field identification and classification literature (Myers, 1991; Fautin and Allen, 1997) and experts on taxonomy (see “Acknowledgments”).
A Mann-Whitney U test (U statistic) (Zar 2009) was used to test for differences in width between anemones with and without ectosymbionts, and a Spearman rank correlation coefficient (rs statistic) (Zar 2009) was used to test for a correlation between anemone width and the number of ectosymbionts.
Despite extensive searches we found only 28 S. gigantea anemones, all in shallow water from 0-1 m deep at low tide on the inner reef flat between Blue Hole and the causeway at Lelu (n=17), and along both sides of the airport runway at Okat Harbor (n=11) (Fig. 1). The anemones all occurred in areas with sandy substrates and seagrasses, but usually beside a hard substrate such as a rock or a dead or living coral. The mean maximum width of the anemones was 21.3 cm (SD, 5.5; range, 12-31 cm).
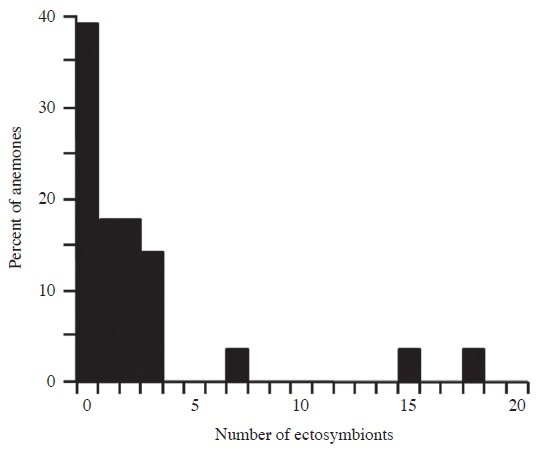
Ectosymbionts (n=67) of seven species associated with 60.7% of S. gigantea. The number of ectosymbionts per anemone was positively skewed, with a mean of 2.4 per anemone (SD, 4.3) and 3.9 per occupied anemone (SD, 5.0; range, 1-18) (Fig. 2). Anemones hosting one or more ectosymbionts did not differ significantly in size (mean, 20.9; SD, 4.9; n=17) from anemones lacking ectosymbionts (mean, 21.9; SD, 6.6; n=11; U=105, p=0.60), and there was no significant correlation between anemone size and the number of ectosymbionts (rs=- 0.02, p=0.92).
Decapod crustaceans (n=35) of four species comprised 52.2% of the ectosymbionts and associated with 39.3% of the anemones, with a mean of 1.3 decapods per anemone (SD, 3.0) and 3.2 decapods per occupied anemone (SD, 4.2; range, 1-15). The squat shrimp Thor amboinensis (de Man, 1888) comprised 20.9% of the ectosymbionts and 40.0% of the decapods, and associated with 10.7% of the anemones, with a mean of 0.5 per anemone and 4.7 per occupied anemone (SD, 5.5; range, 1-11; n=3). Individuals occurred both above and below the oral disc of the anemones (Fig. 3). Unidentified hermit crabs (superfamily Paguroidea) represented 20.9% of the ectosymbionts and 40.0% of the decapods, and associated with 17.9% of the anemones, with a mean of 0.5 per anemone and 2.8 per occupied anemone (SD, 2.2; range, 1-6; n=5). All individuals were under the oral disc of the anemones. The glass anemone shrimp Periclimenes brevicarpalis (Schenkel, 1902) accounted for 9.0% of the ectosymbionts and 17.1% of the decapods, and associated with 14.3% of the anemones (Fig. 4), with a mean of 0.2 per anemone and 1.5 per occupied anemone (SD, 0.6; range, 1-2; n=4). All occurred above the oral disc of the anemones (Fig. 4). A small, unidentified species of crab (infraorder Brachyura) was observed under the oral disc of an anemone, comprising 1.5% of the ectosymbionts and 2.9% of the decapods.
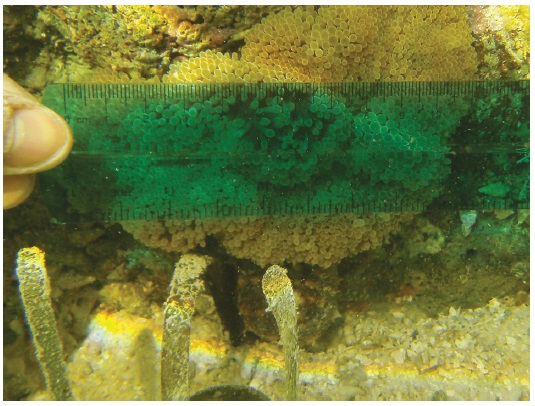
Sea cucumbers of two species comprised 26.9% of the ectosymbionts and associated with 10.7% of S. gigantea, with a mean of 0.6 per anemone and 6.0 per occupied anemone (SD, 8.7; range, 1-16; n=3). The brown curryfish sea cucumber Stichopus vastus Sluiter, 1887 represented 23.9% of the ectosymbionts and 88.9% of the sea cucumbers, and associated with 3.6% of the anemones, with a mean of 0.6 per anemone. We observed 16 small individuals under the oral disc of a single anemone (Fig. 5). The tiger-tail sea cucumber Holothuria hilla Lesson, 1830 represented 3.0% of the ectosymbionts and 11.1% of the sea cucumbers, and associated with 7.1% of the anemones, with a mean of 0.07 per anemone and 1.0 per occupied anemone. Twice we observed a small individual under the oral disc of an anemone (Fig. 6).
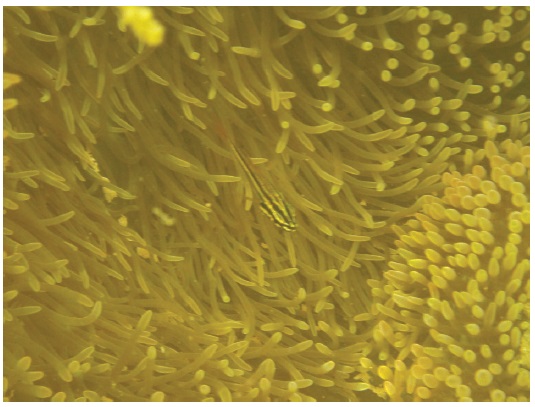
The seven-striped cardinalfish Ostorhinchus novemfasciatus (Cuvier, 1828) represented 20.9% of the ectosymbionts and associated with 25.0% of S. gigantea, with a mean of 0.5 per anemone and 2.0 per occupied anemone (SD, 0.8; range, 1-3; n=7). It occurred above or beside the oral disc of the anemones and often occurred very close to or possibly contacting the tentacles of the anemones (Fig. 7).
Five anemones (17.9%) hosted two species of ectosymbionts. The combinations included (1) 11 T. amboinensis and four hermit crabs; (2) six hermit crabs and one H. hilla; (3) two hermit crabs and an unidentified crab; (4) oneT. amboinensis and two O. novemfasciatus; and (5) 16 S. vastus and two O. novemfasciatus.
This study represents the first quantitative analysis of ectosymbionts associating with S. gigantea. Our lack of a correlation between anemone size and the number of ectosymbionts was also reported for studies of the shrimp T. amboinensis associating with the anemone Stichodactyla helianthus (Ellis, 1768) (see Baeza and Piantoni, 2010), the shrimp Ancylomenes pedersoni (Chace, 1958) associating with the anemone Condylactis gigantea (Weinland, 1860) (see Nizinski, 1989), and the shrimp Ancylomenes holthuisi (Bruce, 1969) associating with the anemone Stichodactyla haddoni (Saville-Kent, 1893) (see Khan et al., 2003). However, a significantly positive correlation between anemone size and the number of ectosymbionts was reported for decapod crustaceans associating with the anemone Telmatactis cricoides (Duchassaing, 1850) (see Wirtz, 1997) and the shrimp Periclimenes rathbunae Schmitt, 1924 associating with the anemone S. helianthus (Hayes and Trimm, 2008).
Only one of the seven species observed in this study, the shrimp P. brevicarpalis, is an obligate commensal of anemones (Bruce and Svoboda, 1983). All other species are facultative associates. Consistent with our observations, others have reported that P. brevicarpalis occurs singly or in pairs (Bruce and Svoboda, 1983; Fransen, 1989; Guo et al., 1996; Khan et al., 2004).
The shrimp T. amboinensis is an associate of many species of sea anemones and other invertebrates (e.g., Fransen, 1987), and often occurs in large groups lacking social structure (Baeza and Piantoni, 2010). Our maximum count of 11 T. amboinensis with S. gigantea equaled the maximum counts of T. amboinensis with the anemones S. helianthus (Baeza and Piantoni, 2010) and Stichodacytla tapetum (Hemprich and Ehrenberg in Ehrenberg, 1834) (Guo et al., 1996). Wirtz (1997) reported up to 18 T. amboinensis associating with the anemone T. cricoides.
Both P. brevicarpalis and T. amboinensis were reported to associate with and occasionally cohabit with S. gigantea in Indonesia, but no data on the frequency of association were provided (Fransen, 1989). We did not observe both species cohabiting an anemone. The only other positively identified species of decapod crustacean reported associating with S. gigantea is Petrolisthes ohshimai (Miyaki, 1937) (Dunn, 1981).
Sea cucumbers generally do not associate with anemones (Bakus, 1973). However, Dunn (1981) reported that “there was often a holothurian tucked in under the overhanging oral disc” of S. gigantea in Jakarta Bay, Indonesia. Our observations of H. hilla and S. vastus associating with S. gigantea represent new records of association and the first identified species of sea cucumbers associating with S. gigantea. However, the association may be incidental rather than intentional because these species of sea cucumbers often seek shelter under the nearest overhang or crevice, which is usually a live or dead coral, or a rock (Alexander M. Kerr, personal communication). Evidently S. gigantea also provides a suitable shelter.
Our observation of the cardinalfish O. novemfasciatus associating with S. gigantea represents a new record of association. Only one other species in the genus, Ostorhinchus nanus (Allen, Kuiter, and Randall, 1994), had previously been reported to associate with an anemone (Randall and Fautin, 2002; Arvedlund et al., 2006). Seven other species of fishes have been reported to associate with S. gigantea (Dunn, 1981; Fautin and Allen, 1997). Karplus (2014) listed an eighth species of fish, but it was not reported to associate with S. gigantea in the literature citation provided.
This study documents the first locality records of S. gigantea, P. brevicarpalis, T. amboinensis, and S. vastus for Kosrae. In Micronesia, S. gigantea has been recorded from Yap Island and Ulithi Atoll (Dunn, 1981), P. brevicarpalis has been recorded from Palau (Miyake and Fujino, 1968), Guam (Paulay et al., 2003), and Eniwetok Atoll in the Marshall Islands (Bruce, 1979), T. amboinensis has been recorded from Palau (Miyake and Hayashi, 1966), Ifaluk Atoll in Yap (Chace, 1972), Guam (Paulay et al., 2003), and the Marshall Islands (Bruce, 1989), and S. vastus has been recorded from Palau (Pakoa et al., 2009), Yap (Pakoa et al., 2009), Chuuk (Lee and Shin, 2014), and Pohnpei (Kinch, 2012). None of these species was reported from a comprehensive survey of the marine environment of Kosrae in the late 1970s (Eldredge et al., 1979) or in subsequent surveys of anemones (Hobbs et al., 2013) and sea cucumbers (Kerr, 1994; Kerr et al., 2008; Lee and Shin, 2013). However, S. gigantea was observed in Kosrae during a brief visit in 1992 by anemone researcher Daphne Fautin (personal communication) and S. vastus was recently photographed in Kosrae by Doug Beitz of Kosrae Nautilus Resort (A. M. Kerr, personal communication), but these records were never published.
Several human residents of Kosrae informed us that S. gigantea is often harvested as a source of food, which may explain why it was relatively difficult for us to find it. Des Rochers (1992) listed unidentified anemones among the marine invertebrates harvested for food in Kosrae, but Lambeth and Abraham (2001) did not. Dunn (1981) reported that the people of Jakarta Bay, Indonesia, also eat S. gigantea. Given the diversity of invertebrates and fishes that often associate with S. gigantea (Dunn, 1981; Fautin and Allen, 1997), overharvesting of S. gigantea may have a devastating effect on multiple species of ectosymbionts. We recommend protecting the ectosymbiotic assemblage of S. gigantea by establishing a sustainable system of harvesting.