The Baculovirus Expression Vector System (BEVS) is used extensively for the expression of a variety of recombinant proteins in insect cells (1-3). The BEVS has advantages such as a high capacity for large insert genes and similarity to mammalian cell’s with respect to post-translational modification compared to prokaryotic cells and yeast (4-6). In addition, BEVS can produce large amounts of recombinant proteins and has been used in production at the industrial scale, for applications like gene therapy (4-6).
Osteoblastic differentiation is regulated by bone morphogenetic proteins (BMPs). BMPs belong to the transforming growth factor-beta (TGF-β) superfamily and are essential for osteoblastic differentiation. Recombinant human BMP-2(rhBMP-2) and BMP-7(rhBMP-7) are widely known to affect bone formation, remodeling, growth, proliferation and differentiation of osteoprogenitor cells (7-9). In previous studies, rhBMP-2 or the rhBMP-7 homodimers have been successfully demonstrated to possess biological activities such as promotion of healing bone defects in many animal experiments (9, 10). Interestingly, BMPs heterodimer forms were more potent and biologically active than the homodimer forms in bone induction and osteoblastic differentiation effects (5, 9, 11).
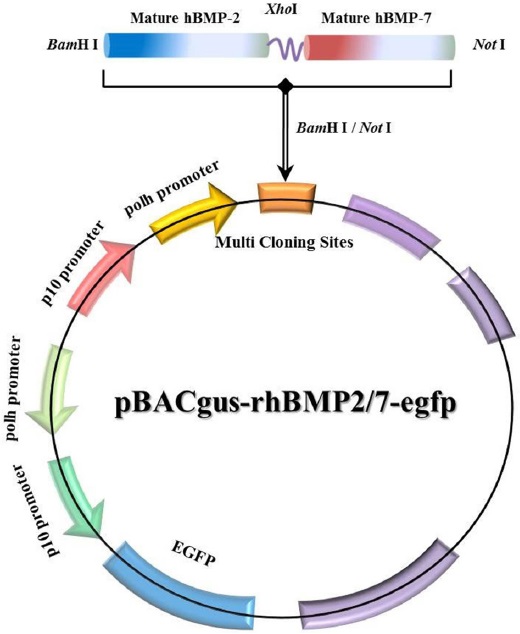
To use the BEVS for producing rhBMP-2/7 protein, the rhBMP-2/7 gene was inserted into the pBAC-gus4x-egfp shuttle vector in direct orientation with respect to the
Sf9 cells were grown in Grace’s insect medium (Gibco, CA, USA) containing 10 % heat-inactivated fetal bovine serum (FBS) (v/v) (Gibco) and 1 % gentamycin (w/v) in an incubator at 27℃. The mouse osteoblast cell line C2C12 was grown in Dulbecco’s Modified Eagle’s Medium (Gibco, CA, USA) supplemented with 10 % fetal bovine serum (FBS) (v/v) (Gibco), 1 % gentamycin (w/v) and was maintained in a humidified incubator with 95 % air and 5 % CO2 at a temperature of 37℃.
The fragments sizes of mature forms of rhBMP-2 and rhBMP-7 are 345-bp and 417-bp, respectively (9). We used specific primer pairs and
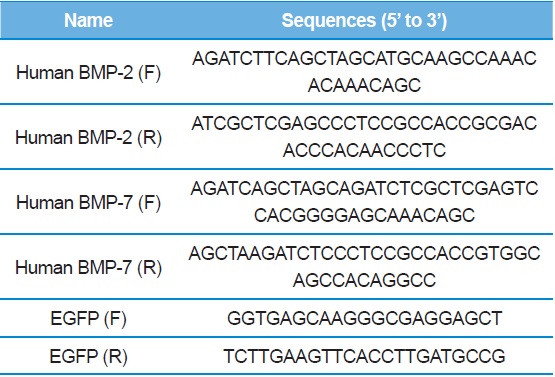
[Table 1.] PCR primer sequences
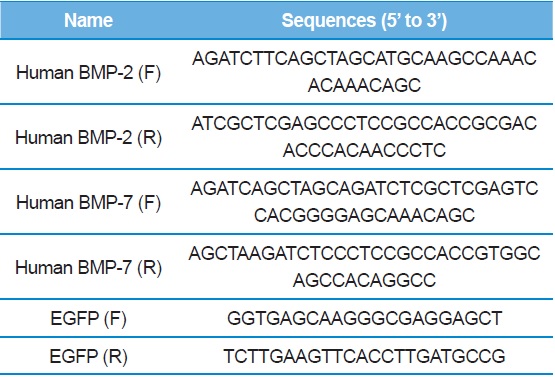
PCR primer sequences
>
Recombinant baculovirus production
For the production of recombinant baculovirus, Sf9 cells were seeded in a 6-well tissue culture plate at 105 cells/well with 2 mL of TNM-FH insect Medium (Welgene, Korea) containing serum and antibiotics. The cells were incubated overnight at 27℃ to allow the cells to attain 70~80 % of morphology and attach to the bottom of the plate. On the next day, the baculovirus genomic DNA and the recombinant pBAC-gus-rhBMP-2/7-egfp shuttle vector were transfected into Sf9 cells using Cellfection®II Reagent (Invitrogen) according to the manufacturer’s instruction. The transfected cells were incubated for at 27℃ until cells showed cytopathic effect (CPE) by 72 h. Subsequently, 2 mL of culture medium from each well was collected and centrifuged to remove the cells and debris.
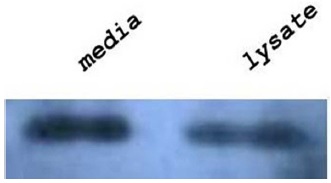
Samples were prepared for western blot analysis in the following manner. Sf9 cells were infected with the pBAC-gusrhBMP rhBMP-2/7-egfp recombinant baculovirus in 6-well plates. After 72 h, cells were collected and lysed in 1× Laemmli buffer [2 % sodium dodecyl sulfate (SDS), 5 % 2-mercaptoethanol, 10 % glycerol, 125 mM Tris, 0.001 % bromophenol blue, pH 6.8]. The 6× His monoclonal antibody was used to detect rhBMP-2/7 protein (Clontech Co., Japan). The protein levels were detected using the ECL western blotting analysis system (Amersham Pharmacia Biotech. Ltd., UK).
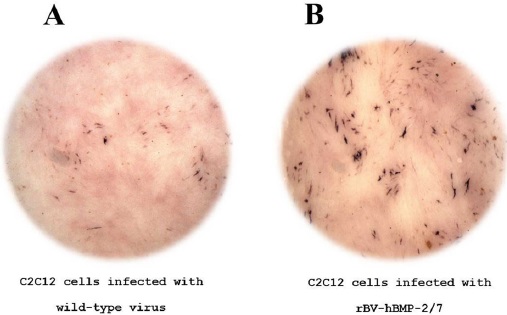
To demonstrate the biological effect of rhBMP-2/7, C2C12 cells were treated rhBMP-2/7 in a 96-well white-tissue culture plate. Untreated C2C12 cells were used as control. Cells were first washed thrice with phosphate-buffered saline (PBS) and fixed using absolute ethanol for one min. The fixed cells were then washed thrice with double distilled, stained using crystal violet solution (Sigma-Aldrich) for 5 min and washed five times with doube distilled water to remove the residual dye. ALP activity was visualized using the Sigma Fast BCIP/NBT substrate (Sigma-Aldrich). We observed the cells using a fluorescence stereomicroscope (Leica MZ16FA, Leica) to examine osteogenic differentiation and recorded our findings using the Leica camera (Leica DFC490, Leica).
To generate the rhBMP-2/7 expressing plasmid DNA for induction of osteoblastic differentiation, we designed the rhBMP-2/7 heterodimer protein with four glycine residues between rhBMP-2 and rhBMP-7 proteins to allow free bond rotation of domains. The rhBMP-2/7 gene was amplified and cloned into the pGEM-T vector and cloning was confirmed by sequencing analysis. As a result, the full-length 762-bp cDNA of rhBMP-2/7 was demonstrated. Subsequently, the rhBMP-2/7 and the
Recombinant baculovirus was obtained by a homologous recombination strategy. Herein, we infected Sf9 cells with rBV-egfp as the wild-type control or with rBV-rhBMP-2/7-egfp as the recombinant virus. After 72 h, the genomic DNAs from baculovirus infected Sf9 cells was amplified using specific primers (BMP-2 F, BMP-7 R, EGFP F/R) to demonstrate the specific viral genome expression pattern of the recombinant baculovirus. The
To confirm the expression of rhBMP-2/7 protein from the recombinant virus genome incorporated into the virus particles, we infected Sf9 cells with recombinant baculovirus for 72 h. Subsequently, we separated the media, infected cell lysate, and pellet from recombinant baculovirus infected cells. Two parts of the samples were analyzed by western blotting using 6× His monoclonal antibody (Fig. 2). Our western blot results show that rhBMP-2/7 was expressed in all parts of the infected cells. Moreover, rhBMP-2/7 was especially expressed in the pellet sample. Next, we investigated the biological activity of the rhBMP-2/7 heterodimer by measuring the effect of secreted rhBMP-2/7 protein as an osteogenic factor on differentiation of C2C12 cells. To confirm the biological role of the recombinant proteins, we infected C2C12 cells with rhBMP-2/7 expressing virus or with wild-type virus as a control. We then used the bone-specific marker, ALP, to evaluate bone differentiation. After 3 d, ALP staining in cells was visualized using Sigma Fast BCIP/NBT. As shown in Fig. 3, we confirmed that osteoblastic differentiation potential of the recombinant baculovirus expressing the rhBMP-2/7 was higher than that of the control wirus. These results suggest that rhBMP-2/7 demonstrates the proper effect on osteoblastic differentiation in C2C12 cells.
Our results demonstrate the role of the potential and biological function of the rhBMP-2/7 heterodimer protein using BEVS to increase the expression of osteoblast-specific genes in mouse pluripotent stem cells during osteogenic differentiation. BMPs are growth factors that have been used in various bone-related studies and clinical applications (7-9). However, several limitations are encountered in the therapeutic application of BMPs, such as requirement of relatively high concentrations, short half-lives, and high cost (9). To overcome these problems, we developed the rhBMP-2/7 heterodimer protein with four glycine residues between rhBMP-2 and rhBMP-7 proteins to facilitate free bond rotation of domains. Previous studies have reported that heterodimer forms of BMPs are more efficient than BMP homodimer forms in bone induction and osteoblastic differentiation effects (5, 9, 11). Therefore, we generated a recombinant baculovirus expressing the human BMP-2/7 protein. Mouse myoblastic C2C12 cells were infected with rhBMP-2/7 to confirm the biological activity of rhBMP2/7. The production of rhBMP-2/7 heterodimer protein using BEVS has more advantages and is distinct from other previous studies. Our results indicate that rhBMP-2/7 shows its biological activity as an osteoblastic growth factor. The development of recombinant baculovirus expressing the rhBMP-2/7 heterodimer by BEVS could be a useful research tool with significant clinical potential and applications for bone regeneration and skeletal development. Further studies examining recombinant rhBMP-2/7 protein production by BEVS will be provide enhanced functionality and efficiency for tissue engineering of bone tissue substitutes.