Basket stars (Echinodermata, Ophiuroidea) seem to be capable of supporting large number of copepods. Humes (1973) could collect 27,209 specimens of copepods from three basket stars of
Basket stars live common in the southeastern coast of Korea and are frequently caught with fishing nets. The authors have been examined the basket stars caught as fisheries bycatches for copepods, but repeatedly failed to find any, certainly due to their detachment from the hosts during the haul of nets. However, recent trials yielded some copepod material of a new species which allow them to describe it herein.
Basket stars (class Ophiuroidea, order Euryalida), the hosts of the examined copepods, were collected as fisheries bycatches from fishing boats. These hosts were fixed with absolute ethanol immediately after the collection. Later, in the laboratory copepod material were obtained from washings of these hosts and preserved in 80% ethanol. Prior to microscopic observation and dissection, copepod specimens were immersed in lactic acid for at least 10 minutes. Dissection and observation were done following the reversed slide method (Humes and Gooding, 1964). All illustrations were drawn with the aid of a drawing tube mounted on microscope. The terminology for the setae of maxilla follows that of Humes and Boxshall (1996). The intact type specimens have been deposited in the National Institute of Biological Resources (NIBR), Incheon, Korea. Descriptions of species were made on the basis of the dissected and figured paratypes. In the description of species body length was measured from the anterior apex of the cephalothorax to the posterior margin of caudal rami, excluding caudal setae. In the formula for the armature of legs 1-4, Roman numerals indicate spines and Arabic numerals represent setae.
>
Telestacicola turgipes n. sp. (
Material examined. 9♀♀, 1♂ from external washings of 3 basket stars,
Other material examined. 4♀♀, 17♂♂ (1♀, 1♂ dissected) from external washings of 10 basket stars,
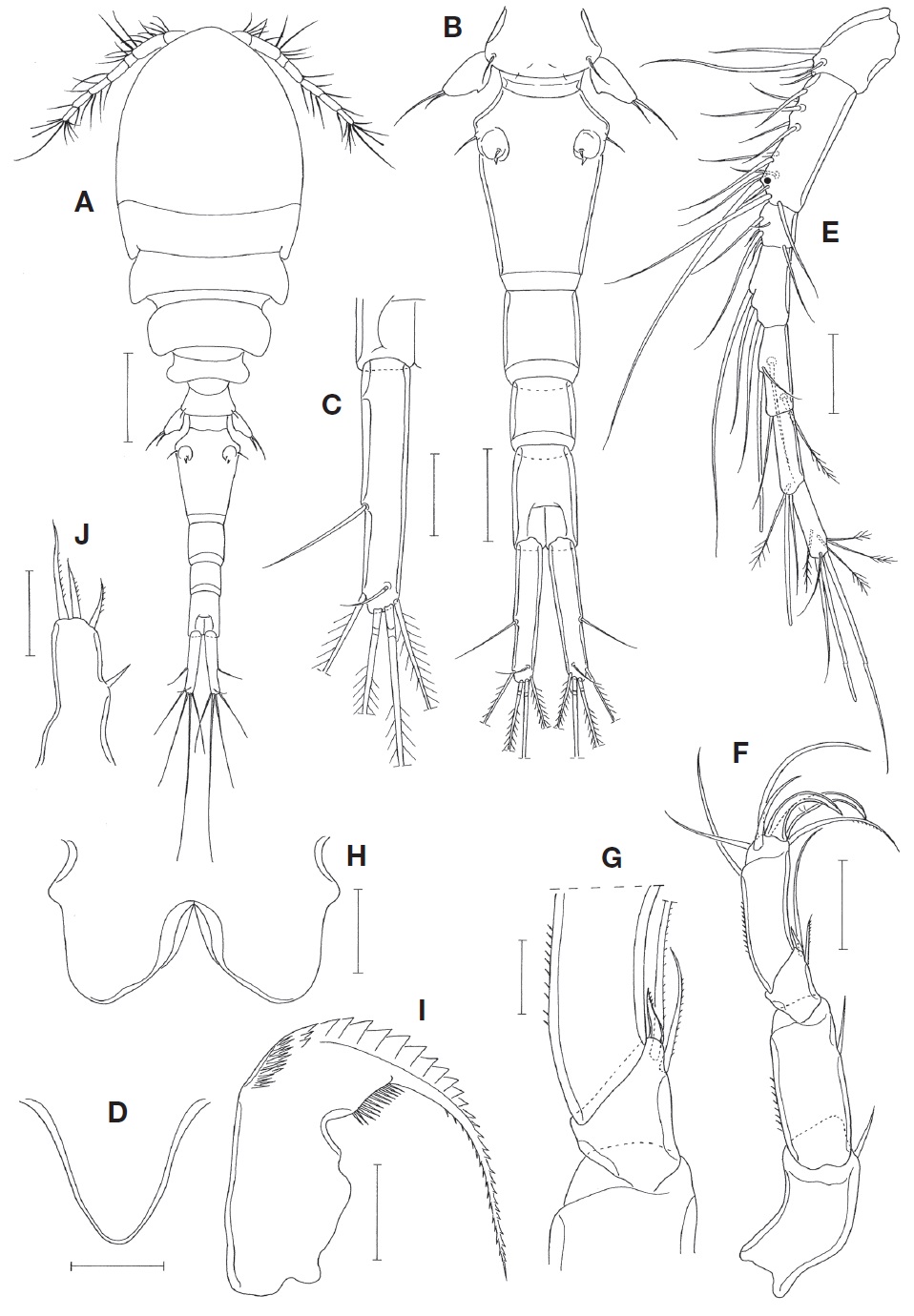
Female. Body (Fig. 1A) slender, 1.50 mm long (1.44-1.61 mm), based on 6 specimens. Figured and described specimen 1.51 mm long. Prosome 810 μm long. Cephalothorax 513×415 μm, longer than wide, with faint dorsal suture line delimiting cephalosome and first pedigerous somite. Second pedigerous somite with slightly projected posterolateral corners. Urosome (Fig. 1B) 5-segmented. Fifth pedigerous somite 125 μm wide. Genital double-somite 221×144 μm, 1.53 times as long as wide, evenly tapering from broadest proximal 0.2 region to distal; with angular lateral apex at broadest region; genital aperture located dorsally at proximal 0.3 region of double-somite. Three abdominal somite 400×83, 75×70, and 108×67 μm, respectively. Anal somite unornamented, with smooth ventro-distal border. Caudal rami slightly divergent. Each ramus (Fig. 1C) 154×30 μm (length/width ratio 5.13 : 1), with 6 setae; outer lateral seta naked and located at 0.58 region of caudal ramus length; 4 distal setae plumose, and small dorsal seta naked.
Rostrum (Fig. 1D) tapering, slightly wider than long, with rounded distal apex. Antennule (Fig. 1E) slender, 345 μm long, 7-segmented; armature formula: 4, 13, 6, 3, 4+aesthetasc, 2+aesthetasc, and 7+aesthetasc; plumose setae 1 on fifth and sixth segments each, and 4 on terminal segment; aesthetascs on distal segments slender, confusable with setae. Antenna (Fig. 1F) 4-segmented. Coxobasis (first segment) with 1 distal seta. Endopod 3-segmented; first segment with 1 subdistal seta and ornamented with fine spinules on outer margin; short second segment with 3 setae at inner distal region, distalmost one of them being small and spiniform (Fig. 1G); terminal segment 77×28 μm, about 2.75 times as long as wide, armed with 2 slender, strongly curved spines and 5 setae, and ornamented with fine spinules on outer margin.
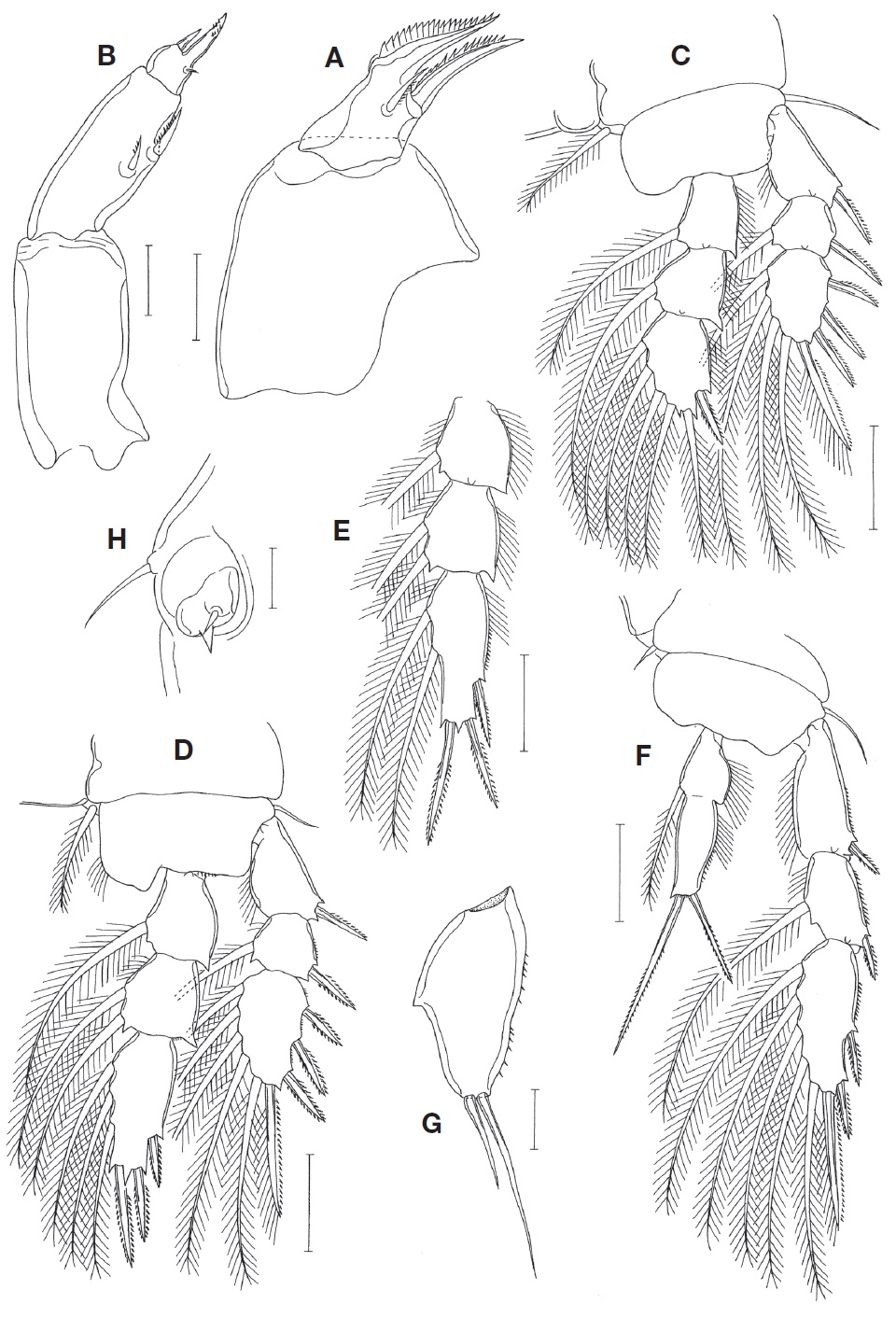
Labrum (Fig. 1H) with broad, smooth posterior lobes; proximal part of lateral margin bluntly produced. Mandible (Fig. 1I) with shallow inner proximal notch; inner margin short and oblique; outer proximal part of gnathobase with 2 rows of spinules followed by more than 10 large teeth; distal lash elongate, with denticles along both margins. Maxillule (Fig. 1J) armed with 4 setae, 3 distal and 1 lateral. Maxilla (Fig. 2A) 2-segmented. Syncoxa (proximal segment) large but unarmed. Basis (distal segment) with short, stiff, tapering lash bearing about 20 denticles along convex outer margin and 2 setae; inner seta (seta I) strong, with spinules along convex outer margin; distal lash and inner seta together giving appearance of pincers; anterior seta (seta II) small and spinulose unilaterally; outer proximal seta (seta III) absent. Maxilliped (Fig. 2B) 3-segmented (syncoxa, basis, and 1-segmented endopod). Syncoxa largest but unarmed. Basis with 2 unequal setae near middle of inner side, both setae unilaterally spinulose. Endopod small, terminated by spiniform process bearing several minute spinules, with 1 spiniform and 1 minute setae.
Leg 1(Fig. 2C), leg 2 (Fig. 2D), and leg 3 with 3-segmented rami. Outer spines on exopods slender. Outer seta on basis of legs 1-4 naked. Leg 3 (Fig. 2E) similar to leg 2, except for having 3 spines and 2 setae on third endopodal segment. Leg 4 (Fig. 2F) with 3-segmented exopod and 1-segmented endopod; inner seta on coxa small and naked; endopod 1-segmented, with indistinct transverse line as articulation vestige; two distal spines 51 μm (outer one) and 88 μm (inner), respectively. Armature formula for legs 1-4 as follows:
Leg 5 represented by 1 dorsolateral seta on fifth genital somite and 1-segmented free exopod. Exopod (Fig. 2G) 73×35 μm, 2.09 times as long as wide, with fine spinules on outer margin, produced inner margin bearing pointed apex in midlength of exopod, and armed with 2 distal setae; outer seta 66 μm long, and inner seta 34 μm long. Leg 6 (Fig. 2H) represented by 1 small seta and 1 tooth-like process in genital aperture and 1 larger seta on lateral margin of double-somite.
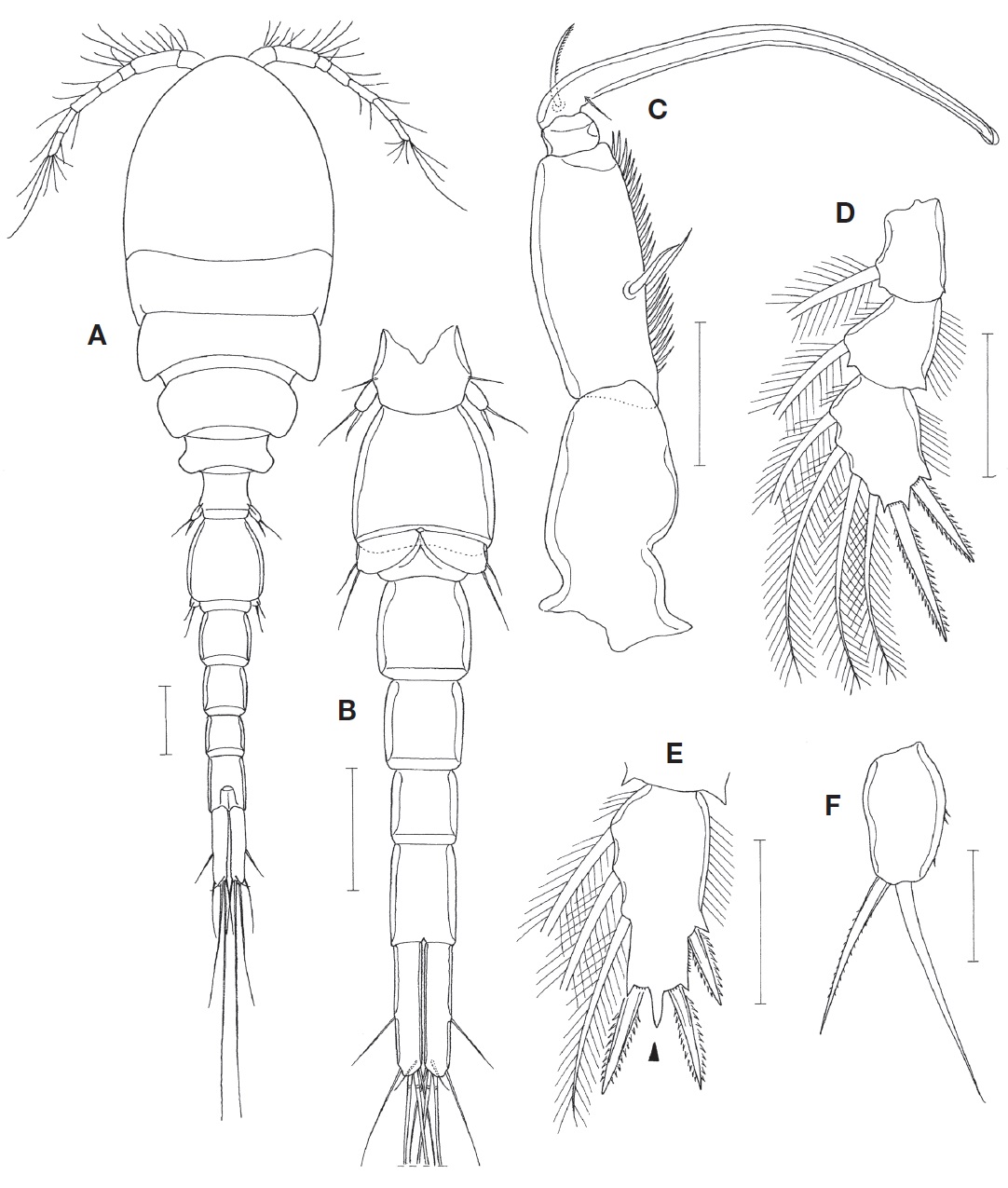
Male. Body (Fig. 3A) resembling that of female, 1.24 mm long. Prosome 625 μm long. Cephalothorax 385×313 μm. Urosome (Fig. 3B) 6-segmented. Fifth pedigerous somite 80 μm wide. Genital somite 140×116 μm, longer than wide. Four abdominal somites 80×77, 72×66, 59×55, and 80×52 μm, respectively. Caudal ramus 113×23 μm, 4.91 times as long as wide.
Rostrum as in female. Antennule as in female, but with 1 additional aesthetasc at distal region of second segment (at place indicated by dot in Fig. 1E). Antenna as in female.
Labrum, mandible, maxillule, and maxilla as in female. Maxilliped (Fig. 3C) 4-segmented. First segment unarmed. Second segment with 2 naked seta at proximal 0.4 region and with 2 rows of spinules along inner margin. Small third segment unarmed. Fourth segment forming long, slender claw, with 1 unilaterally spinulose seta and 1 minute, needle-like seta at proximal region.
Leg 1 endopod (Fig. 3D) with 2 spines and 4 setae on third segment; 2 distal spines 34 μm (outer) and 52 μm (inner) long, respectively. Third endopodal segment of leg 2 (Fig. 3E) with mid-distal process (indicated by arrowhead in Fig. 3E) more pronounced than that of female. Otherwise, leg 1-4 as in female.
Leg 5 exopod (Fig. 3F) small, 25×15 μm, 1.67 times as long as wide, with few spinules on outer margin and 2 distal setae; inner distal seta spiniform, 30 μm long, with fine spinules; outer distal seta 42 μm long. Leg 6 represented by 2 unequal, naked setae on genital operculum (Fig. 3B).
Etymology. The specific name
While describing
While comparing of congeners of
With the removal of
The new species
The seven species of