Since increase in consumption of grain and volatility in grain supply due to global warming, it has highlighted the importance for the food supply as world population is expected to be increase to 9.6 billion in 2050 (UN, 2013). The production of sufficient protein from bovine, poultry meat, and demersal fish represents a serious challenge for the future (van Huis et al., 2015). As an alternative of animal protein, an edible insect is one of the breakthroughs that could solve the food crisis (Rumpold and Schlüter, 2015). According to increased interest in the edible insects, an establishment of the foundation for human or animal food based on a variety of insect resource is ongoing (Belluco et al., 2015). In 2015, cricket has been newly approved as a temporal food ingredient with mealworm beetle, and protaetia brevitarsis seulensis by Korea ministry of food and drug safety. Among the insects, cricket, which is known as high contents of chitin and unsaturated fatty acid, traditionally consumed as a medicine for fever, diarrhea, kidney stone or hypertension as well as a food source (Park, 2001; Ahn et al., 2005). Despite its various aspects, safety evaluation of processed cricket is limited. These characteristics of crickets have motivated the toxicological test herein a 4 wk repeated oral dose toxicity test in rats. Here, we determined that cricket ethanol extract causes no toxicological issues as part of the diet and can serve as an excellent food source, resolving the scarcity of food, in addition to being a possible health supplement in the future.
Preparation of test materials. Cricket, G. bimaculatus collected in the insect farm in Joungsun, South Korea. Crickets were subjected to 3 d defecation period then washed 3 times with distilled water, and then freeze-dried. 1 kg of freeze-dried cricket was homogenized. Sample was soaked into 70% ethanol and extracted by ultrasonicator over 3 times. Extracted sample was filtered using Whatman filter and concentrated by freeze-drying and complete evaporation of solvent. Powder type of sample was dissolved in saline prior to administration.
Animals. Animal experiments were designed and conducted under the Organization for Economic Cooperation and Development (OECD) test guidelines No. 407 ‘Repeated dose 28 d oral toxicity study in rodents’, Good Laboratory Practice (GLP), and the Korean Ministry of Food and Drug Safety (KMFDS) notice no. 2014-136 ‘Toxicity test standards of medicine and medical supplies’ (OECD, 2008). Methods were approved by the Animal Care and Use Committee at the Korea Conformity Laboratory (IA14-01047). Specific pathogen free Crl:CD (Sprague-Dawley) rats were obtained from ORIENTBIO (Sungnam, Korea). Animals were maintained at a standard temperature of 23.3±0.8℃ and a relative humidity of 47.4±5.1 % RH under a 12 h light/dark cycle. Rats were fed a rodent diet (Harlan Teklad, USA). All animals were provided with tap water purified by a reverse osmosis filtering system.
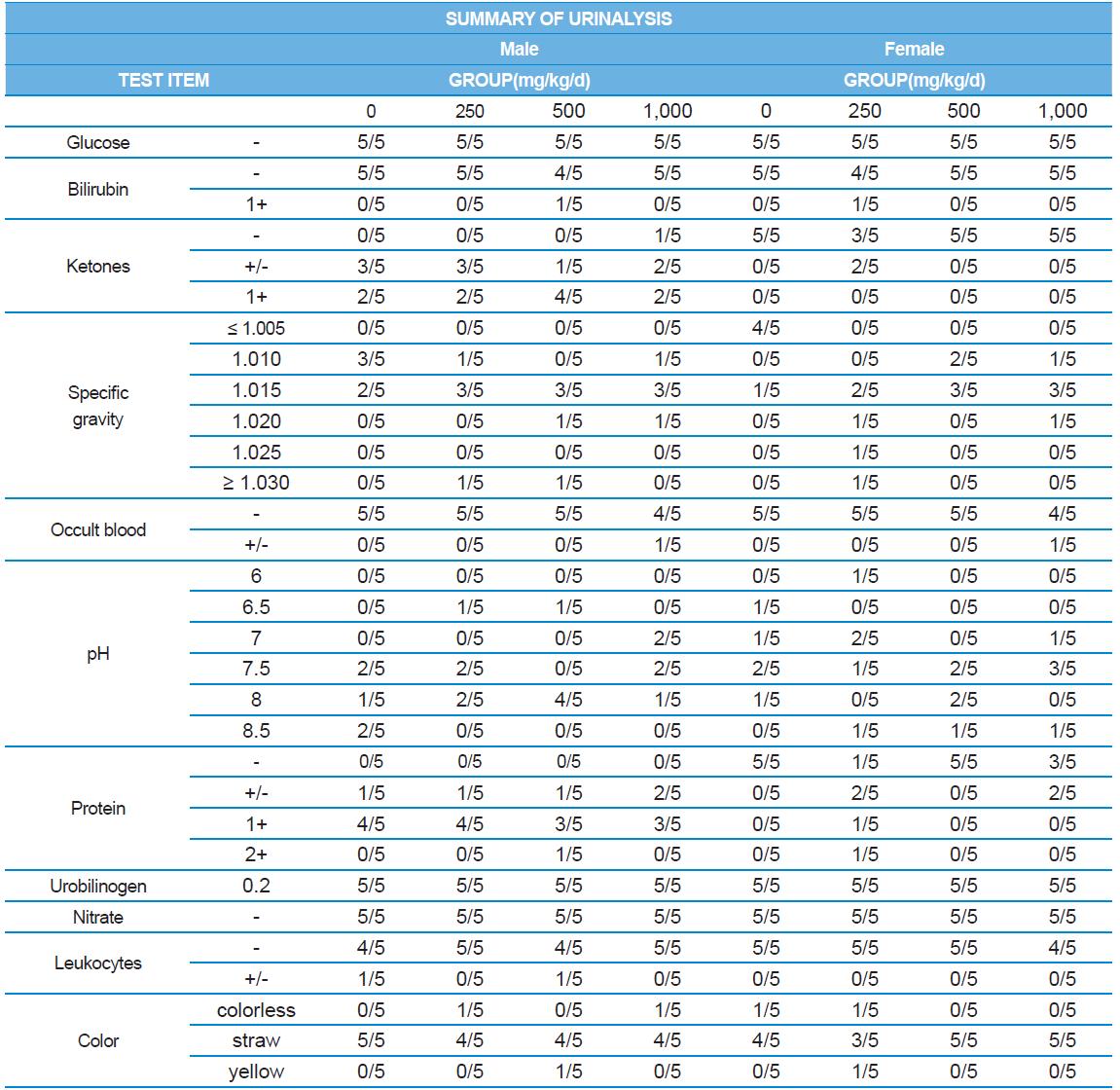
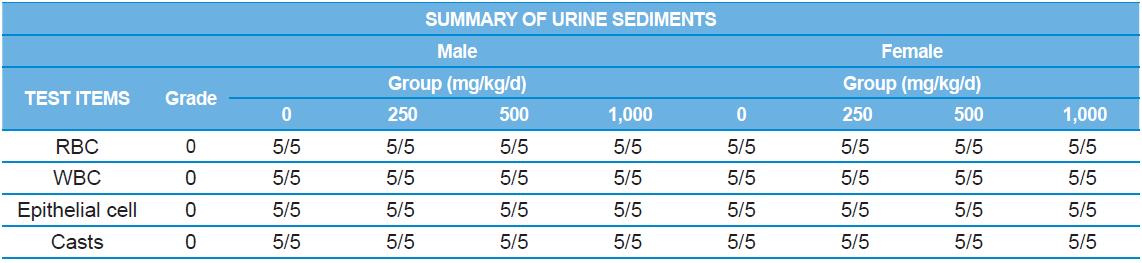
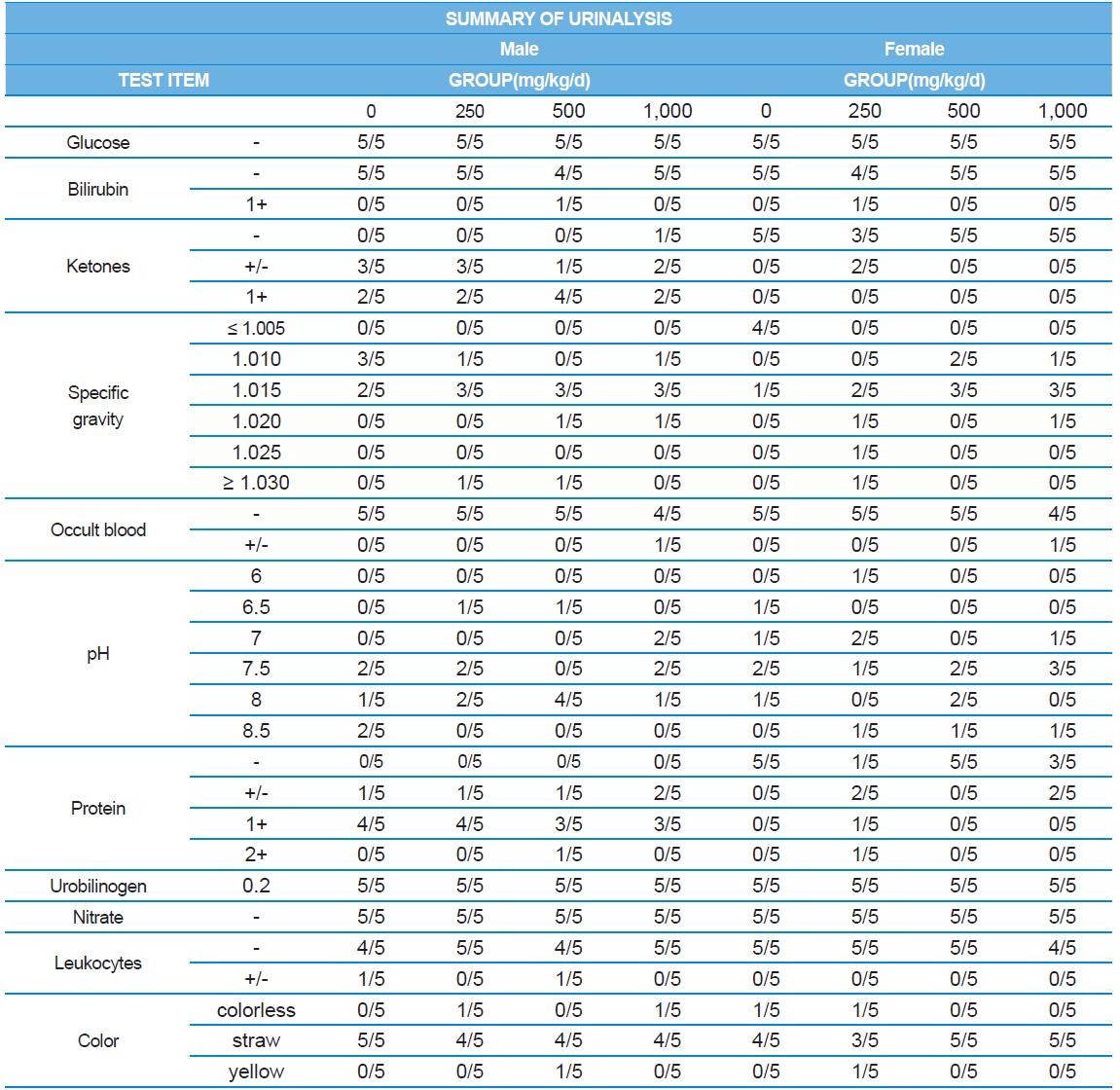
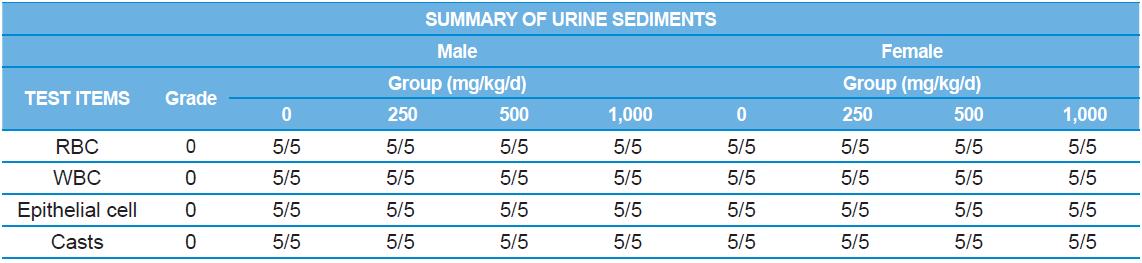
A 4 wk repeated oral dose toxicity study in SD rats. 5-wk-old male and female rats were acclimated for 5 to 7 d prior to administration. When the rats became 6-wk-old, they were exposed to cricket ethanol extract by gavage for 28 d. According to OECD guideline, it is suggested that 1,000 mg/kg as a limit dose of repeated oral dose toxicity study in rodent. Therefore, in the present study, the dose of 1,000 mg/ kg was set to high dose level. Individual dosing volumes were calculated based on 10 mL/kg body weight. During the study, general clinical signs of all treated animals were observed once a day right after administration during the exposure period. Individual animal weight was recorded at acquisition, grouping, right before administration, once a week during the study and before necropsy. Food consumption also measured once a week. On the last week of the study, urine samples were collected from randomly selected 5 animals per each groups. Fresh urine used for analysis using urine test strip (SIEMENS, Germany) and the urine auto-analyzer, Clinitek advantus (SIMENS). Leukocyte, epithelial cell, and cast were counted under microscope.
At necropsy, all animals were laparotomized under isoflurane anesthesia. Blood samples were withdrawn from the abdominal aorta and aliquoted into EDTA-K2 Tube, 3.2 % Sodium citrate Tube, Serum separating tube and Heparin Tube ABGA Syringe(20~30 IU/1 mL).
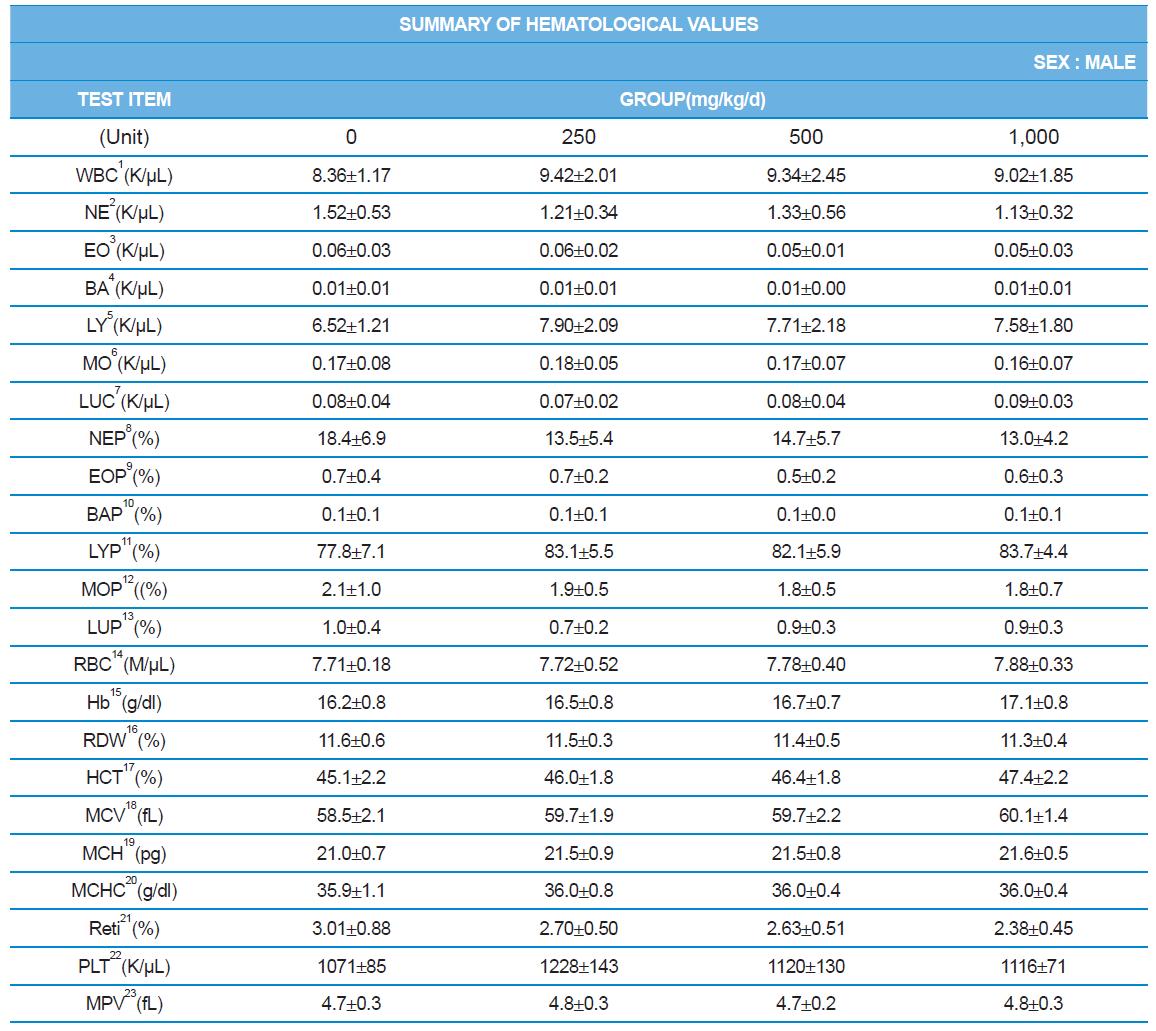
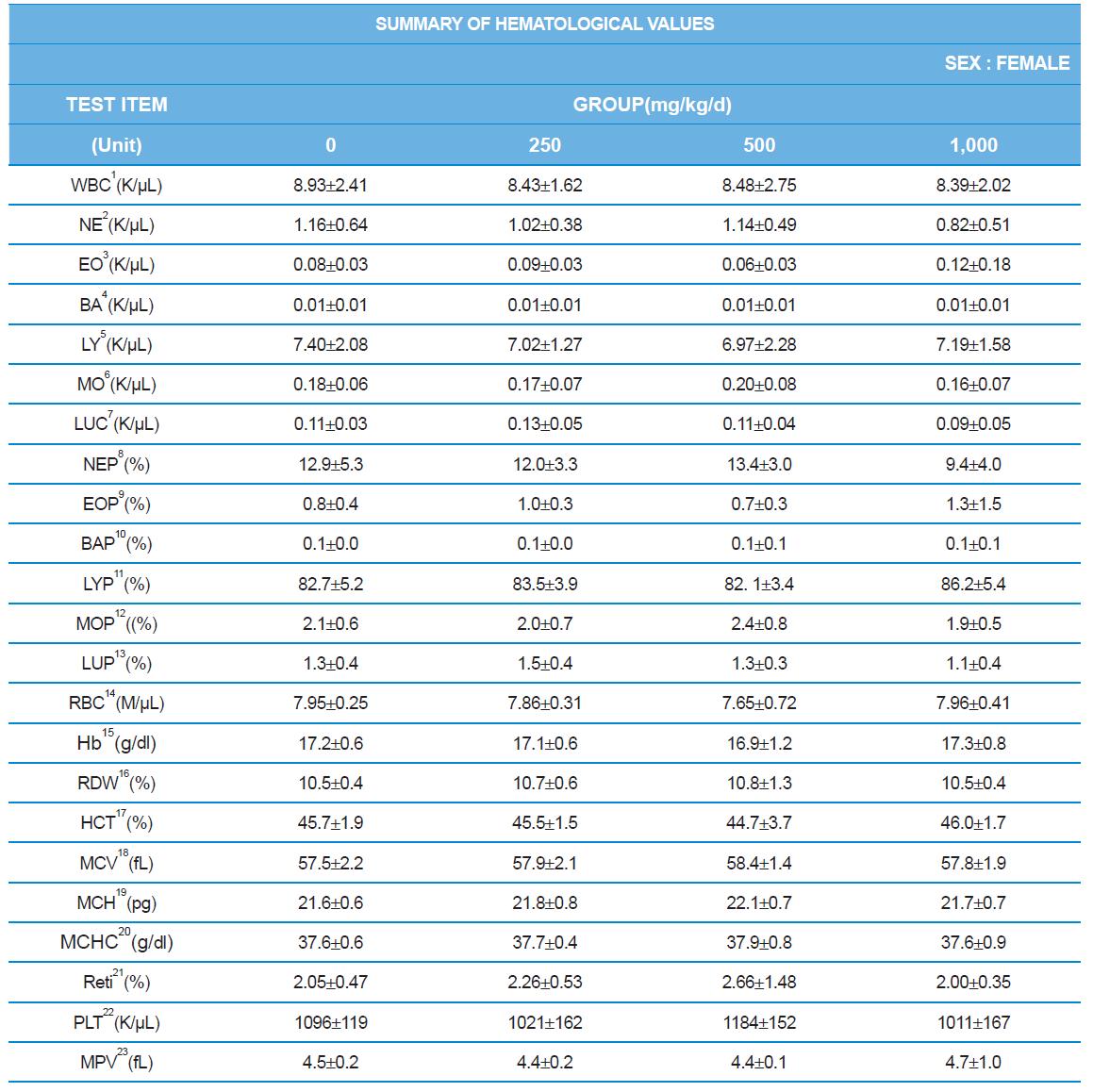
Hematology analyzer, ADVIA 2120 (SIEMENS), blood coagulation analyzer, ACL7000 (Instrumentation Laboratory, USA), biochemistry analyzer, Hitachi7180 (Hitachi, Japan) were used for blood analysis as described by Sung et al. (2014). After complete-mortem examination, organs were weighted and preserved in 10% neutral buffered formalin for histopathological examination.
Statistical analysis. Statistical analysis was carried out using SPSS (Version 12). Statistical evaluation was performed using a two-tailed Student’s t-test or an analysis of variance (ANOVA) following multiple comparison tests with Duncan’s method. Asterisks (*) indicate statistically significant differences compared with the control groups. On day 48, one of the male rats in the 5,000 mg/kg test group was sacrificed at the study director’s instruction due to a technical error by the technical assistant. Only 9 values were used in the male 5,000 mg/kg test group for the statistical analysis of hematology, plasma coagulation, serum biochemistry and absolute/relative organ weight.
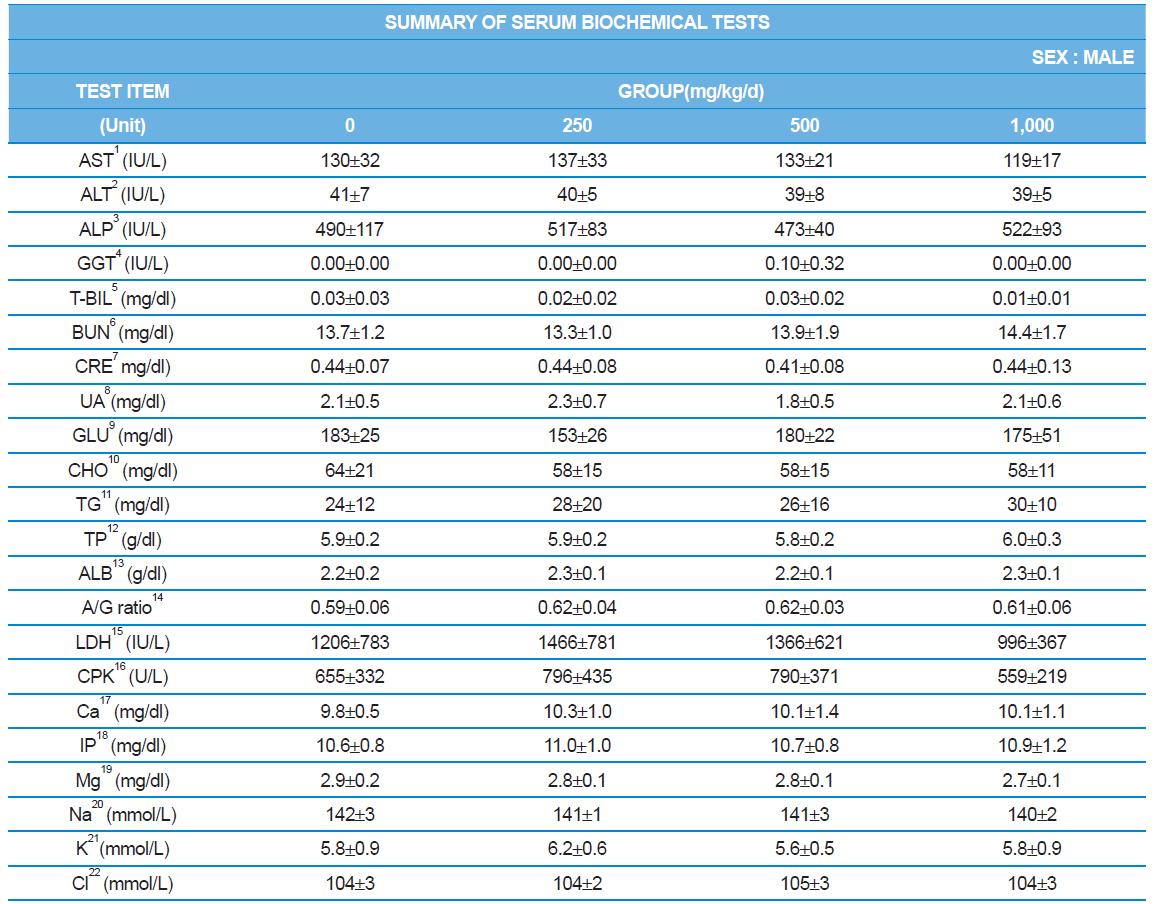
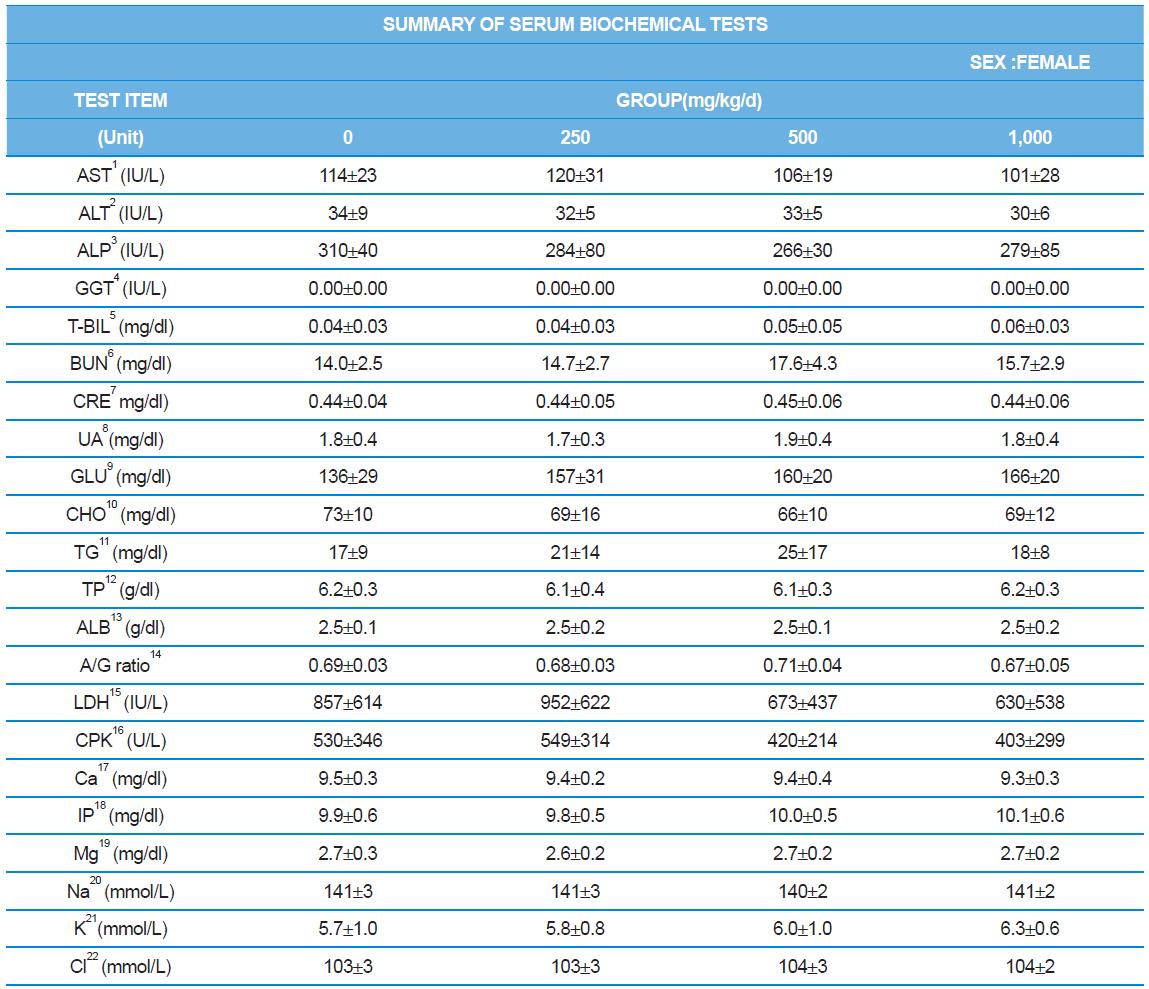
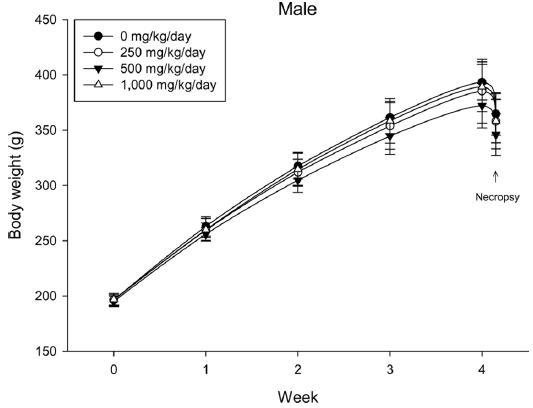
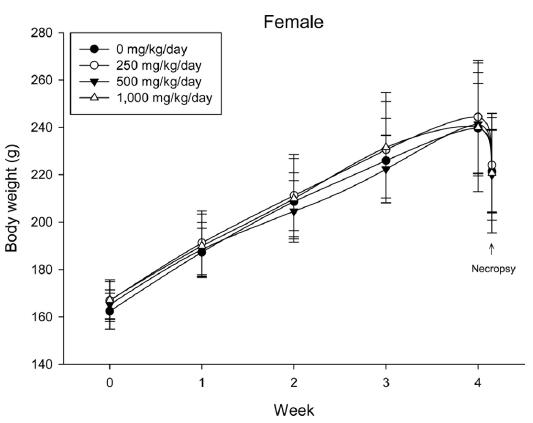
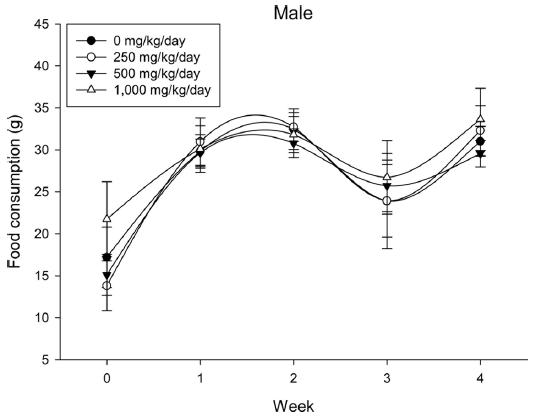
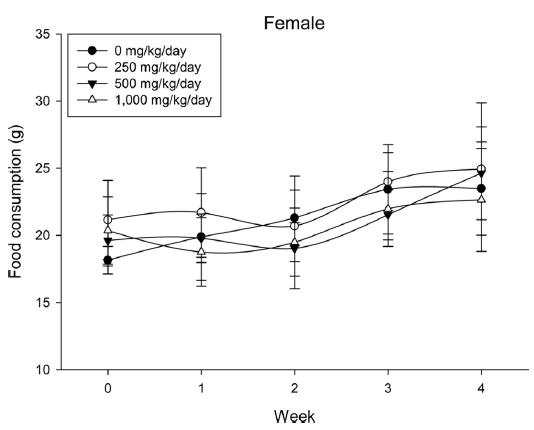
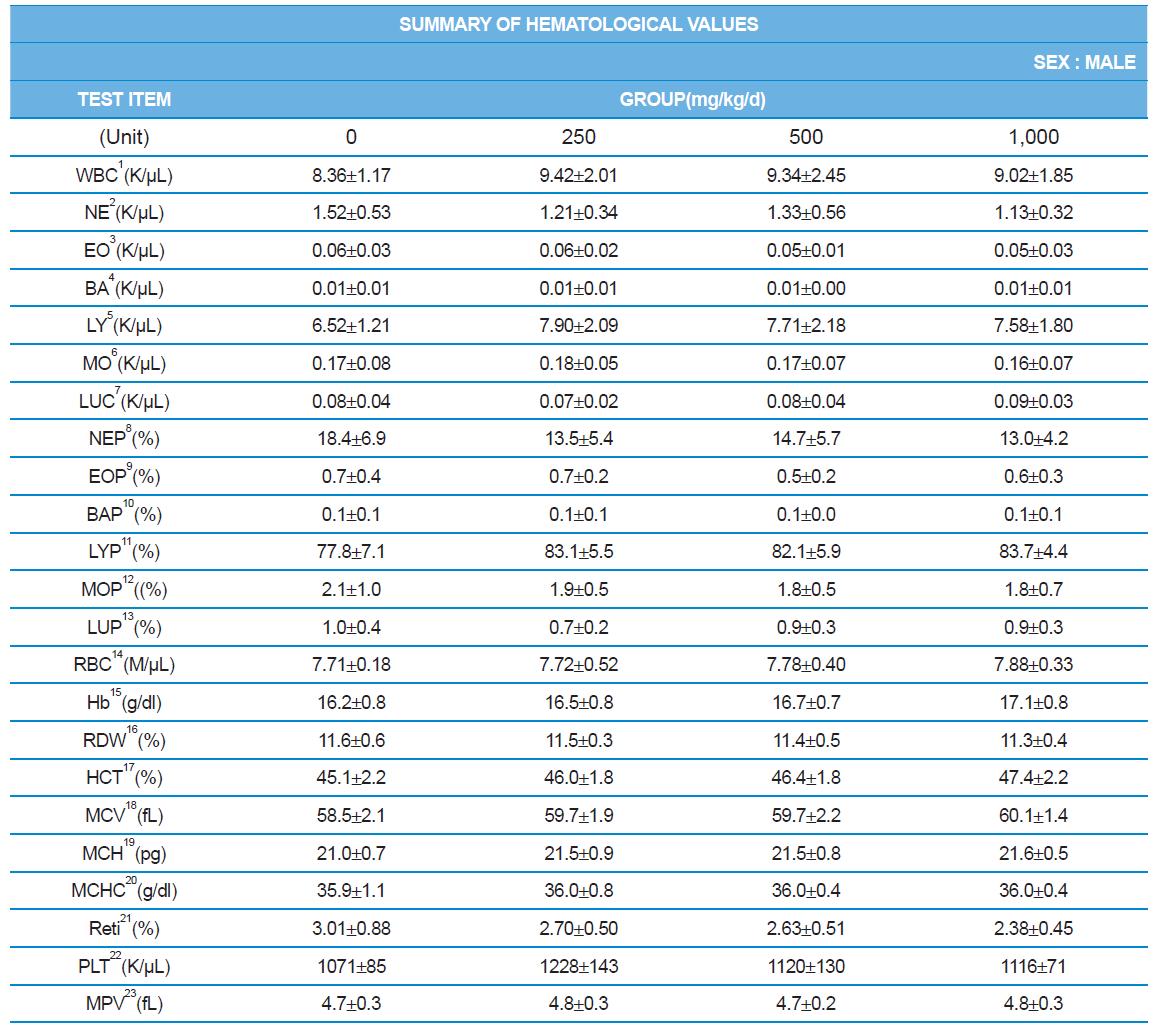
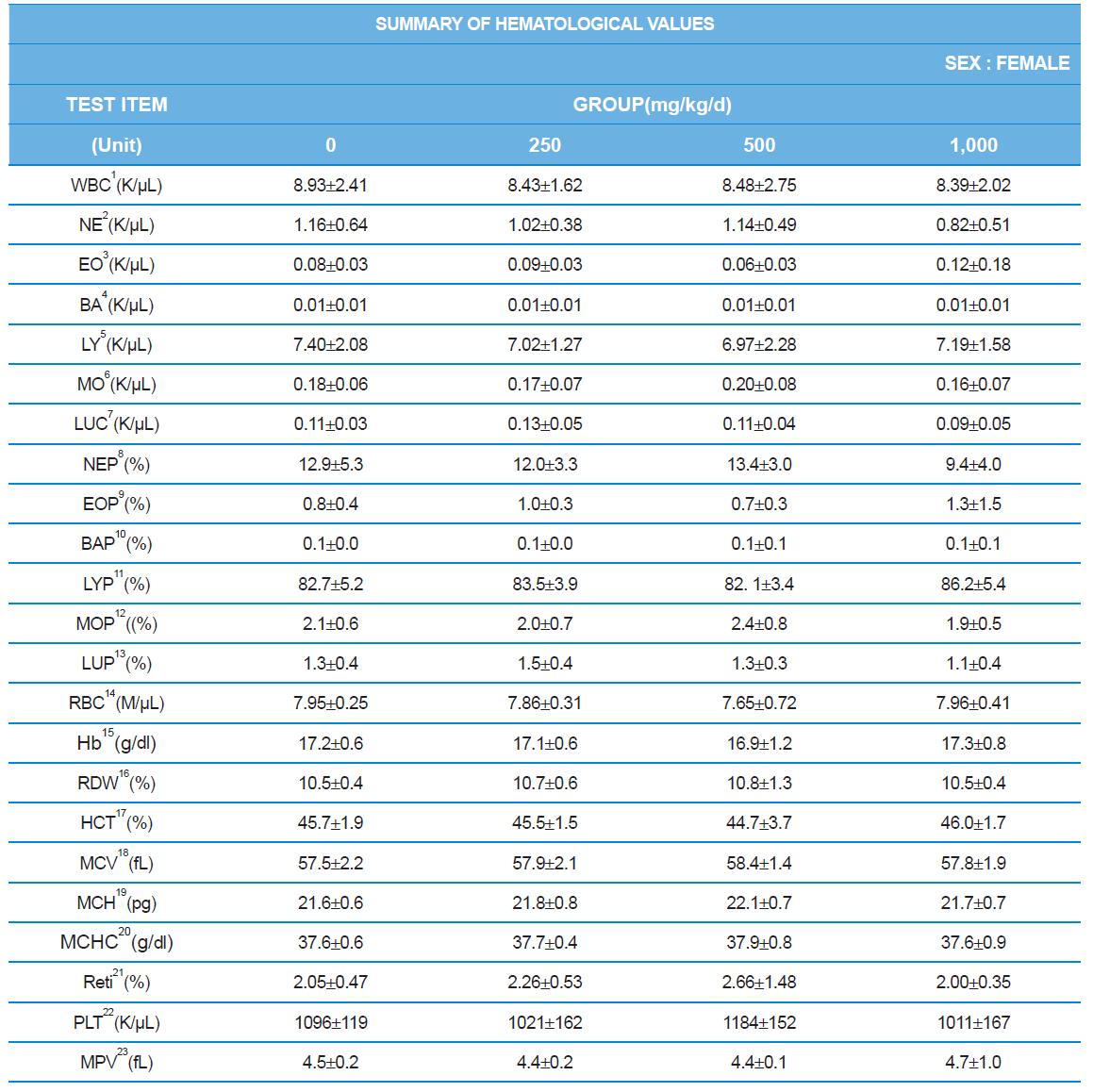
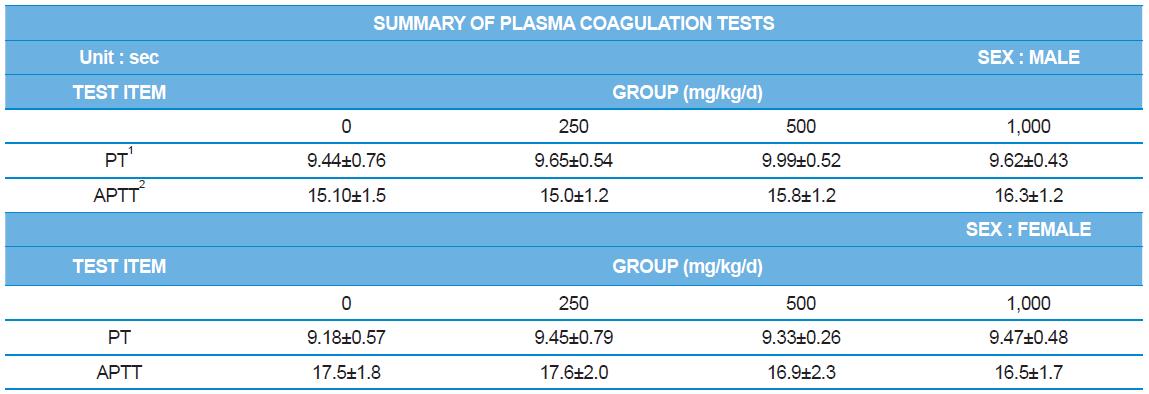
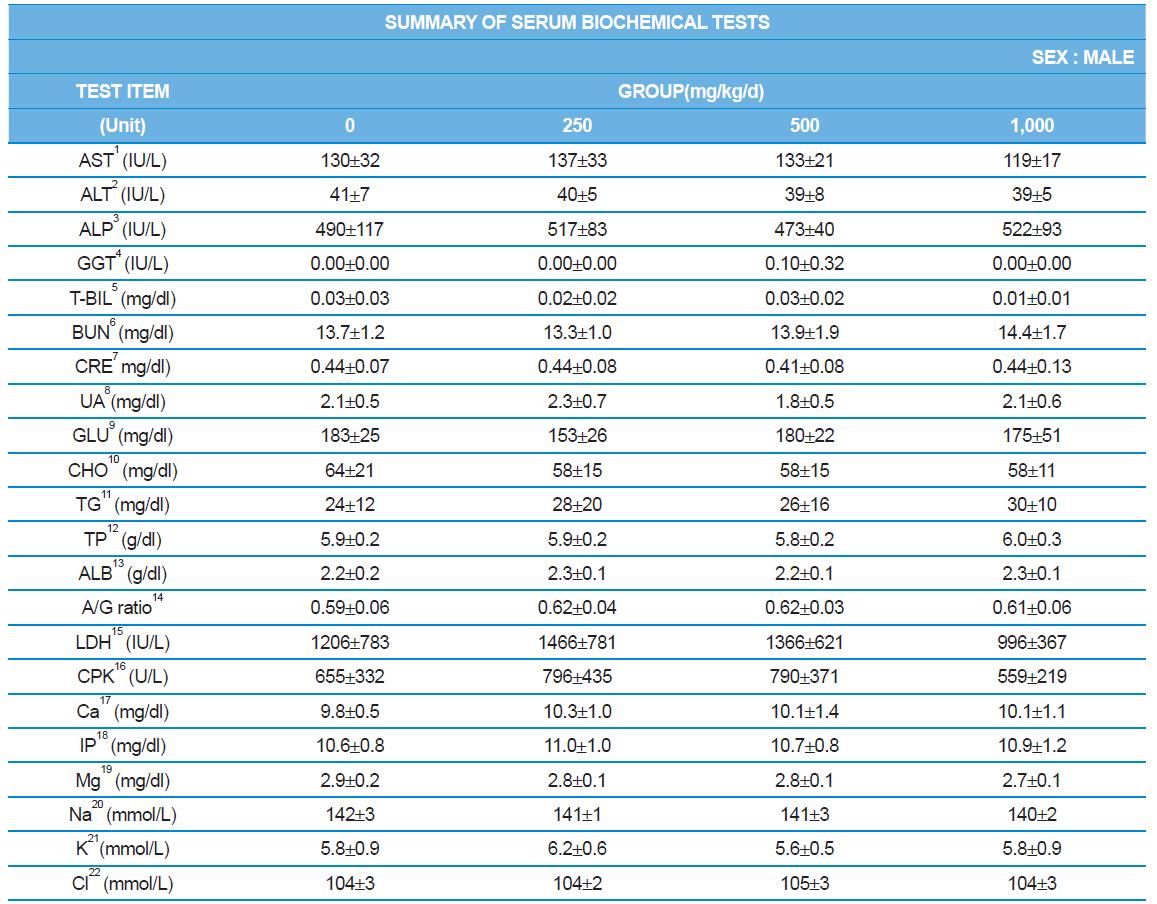
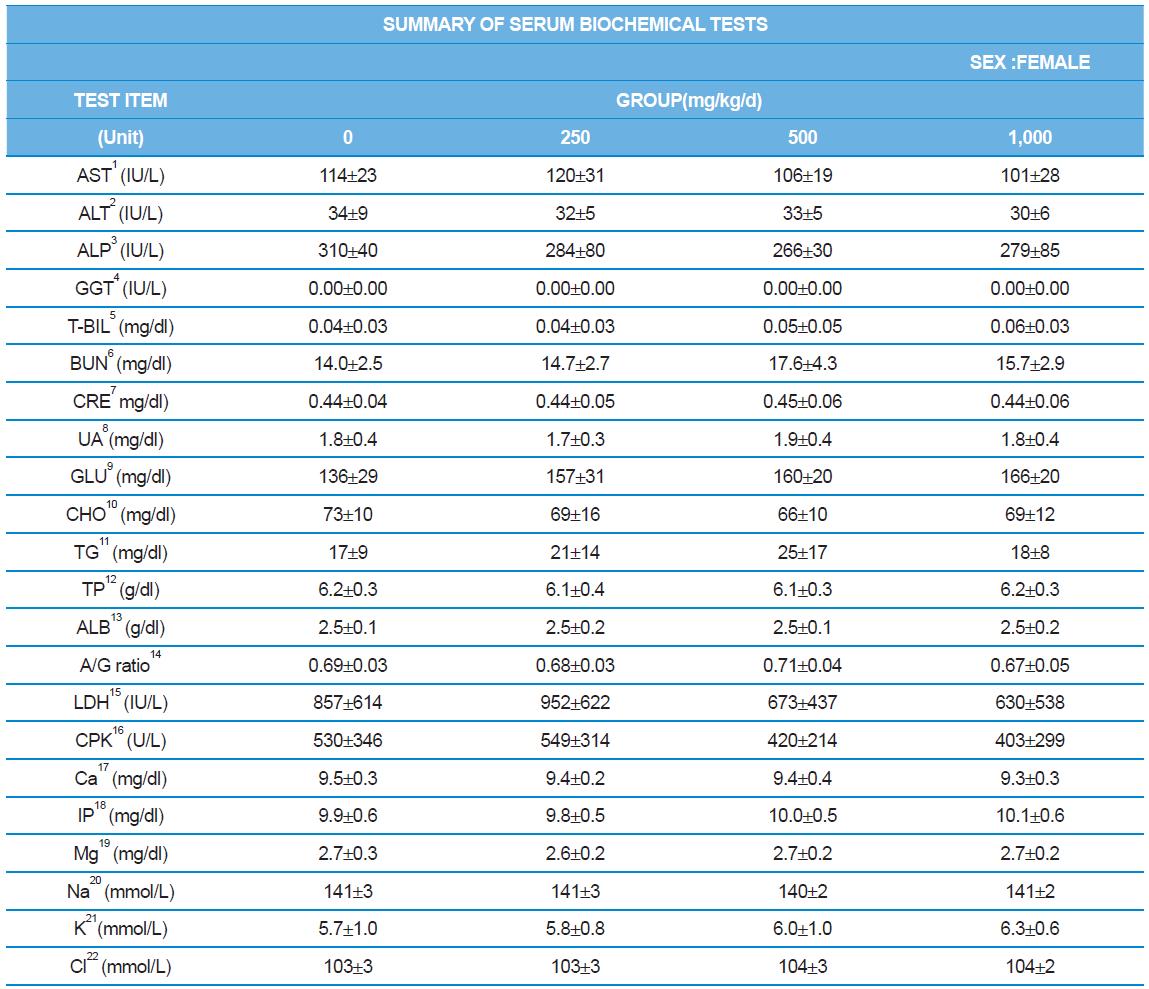
During the 4 wk oral repeated test substance administration of 0, 250, 500 and 1,000 mg/kg doses, body weight changes (Figs. 1-1 and 1-2) and food consumptions (Figs. 2-1 and 2-2) for each sex were measured. There were no significant difference was detected on each week across the vehicle control groups. Morbidity or mortality was not observed in all test groups of both sexes and there were no abnormal clinical signs observed throughout the experimental period. In urinalysis and urine sediment analysis, parameters of treatment and recovery groups of both sexes were found to be comparable with the corresponding control group (Tables 1 and 2). The result of hematological and the plasma coagulation analysis showed no significant difference in either sex in the test groups compared with the control group (Tables 3-1, 3-2, and 4). In serum biochemical analysis, the aspartate aminotransferase, lactate dehydrogenase, and creatine phosphokinase of the male and female rats in 1,000 mg/kg test group was decreased compared with control group (Tables 5-1 and 5-2). However, we did not find any statistical significance in the other test items in the test groups compared with the vehicle control group.
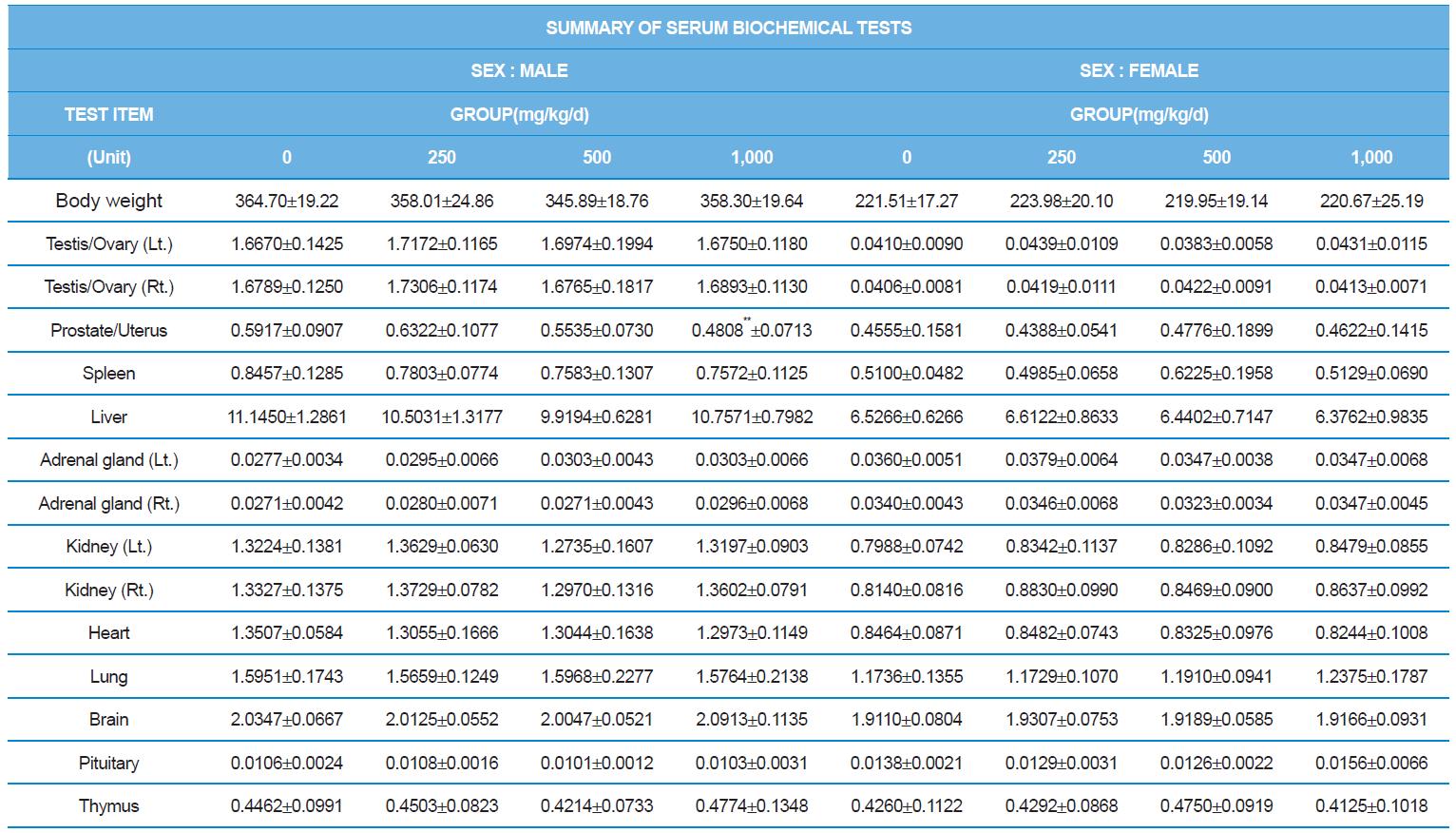
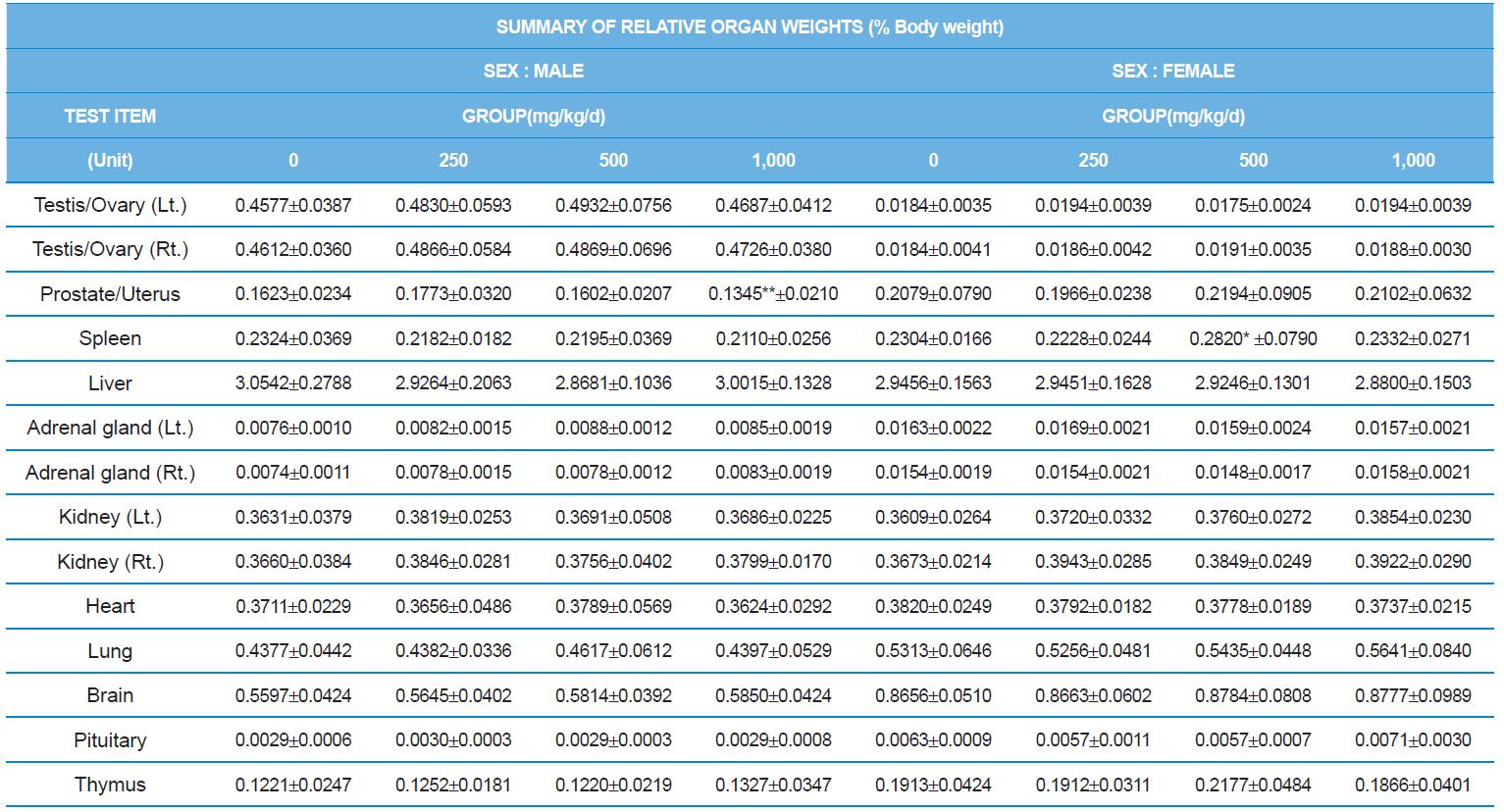
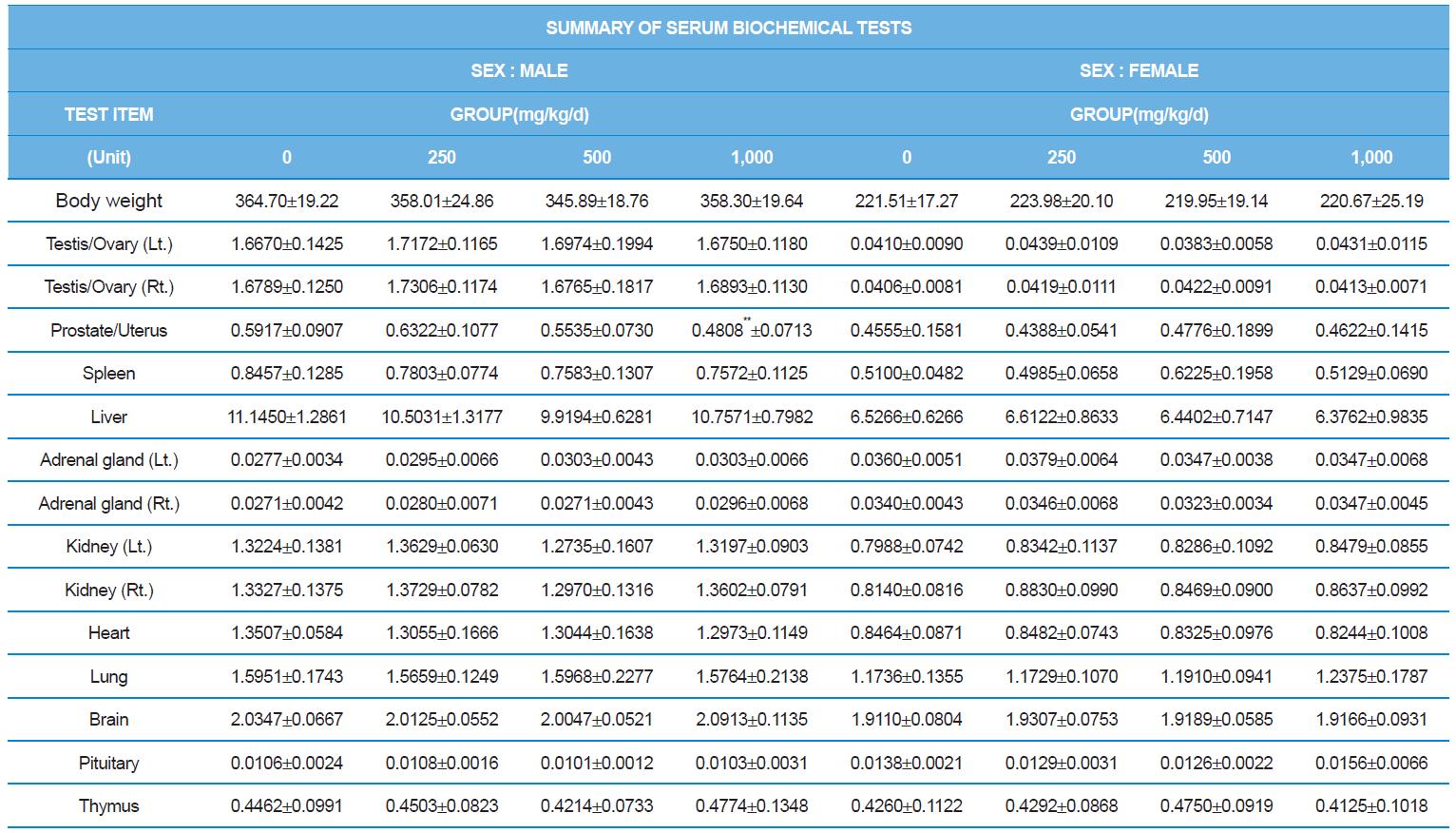
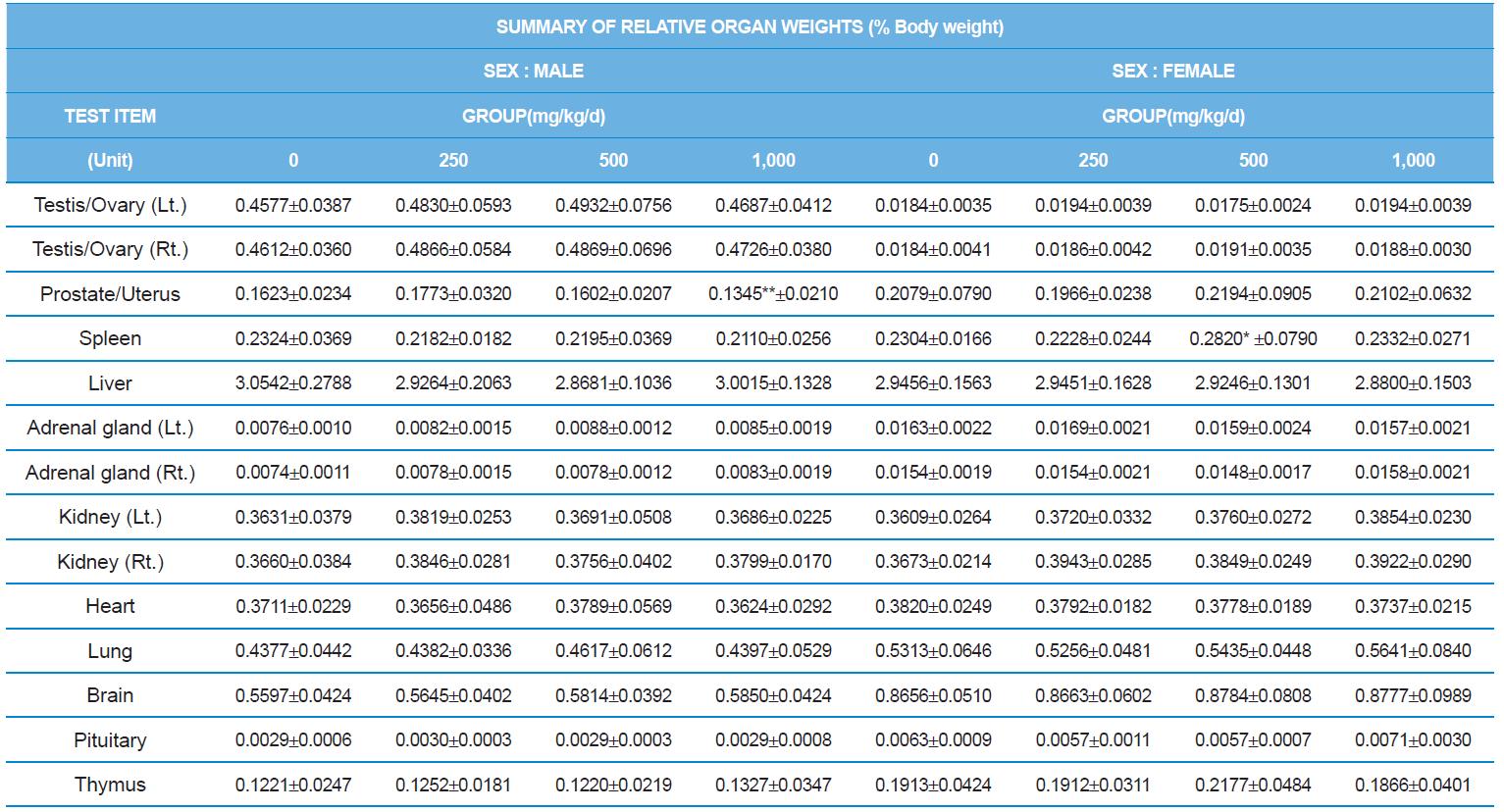
At necropsy, enlargement of spleen was shown in one female rats in 500 mg/kg test group. There was no abnormal gross finding was shown in the other rats. In organ weights, absolute and relative organ weight of prostate in male 1,000 mg/kg test group was statistically significantly decreased compared with the vehicle control group (p<0.01). Relative organ weight of spleen was statistically significantly increased (p<0.05) (Tables 6-1 and 6-2). However, there is no toxicological meaning on these findings: because a) the values were within the normal biological range, b) there was no dose-dependency, and c) the results did not correlate with other analysis results.
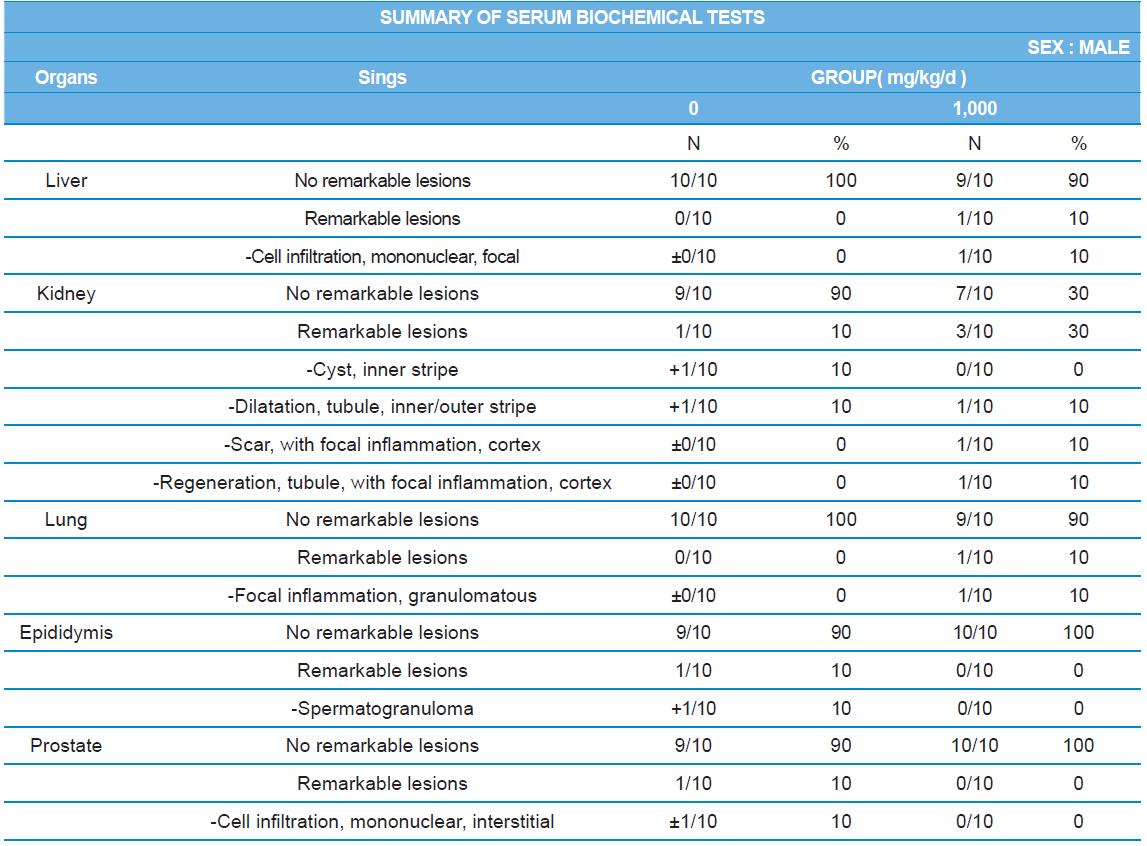
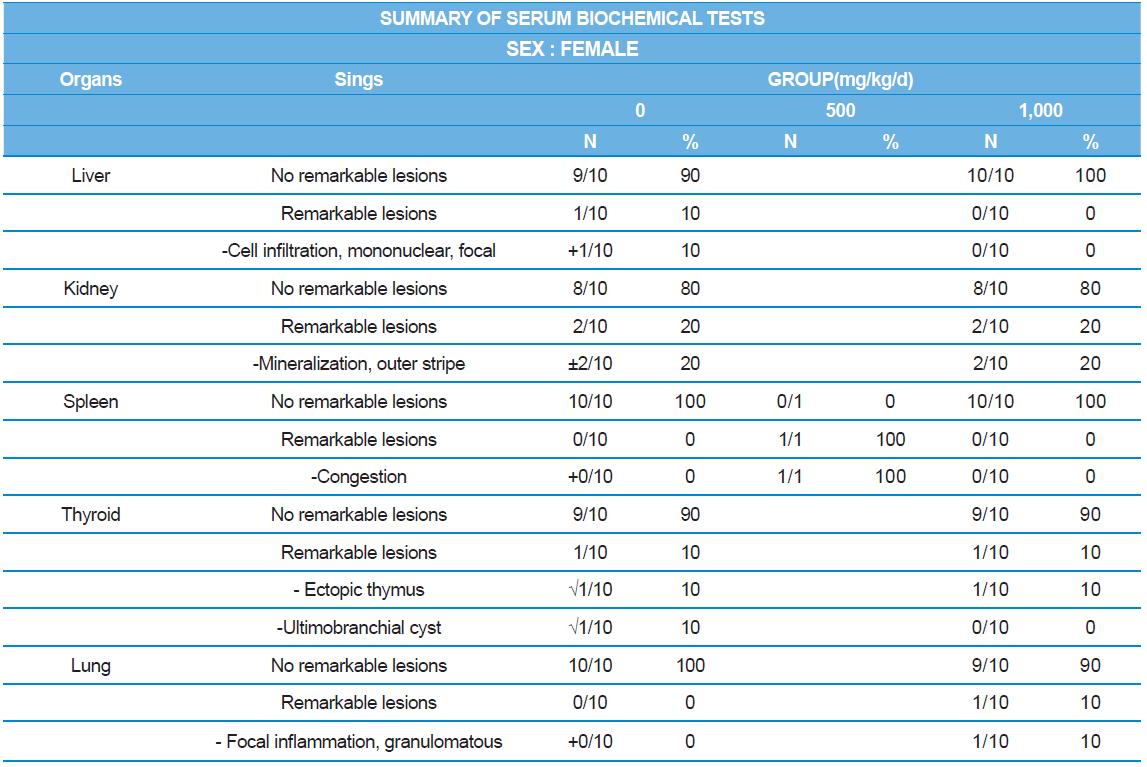
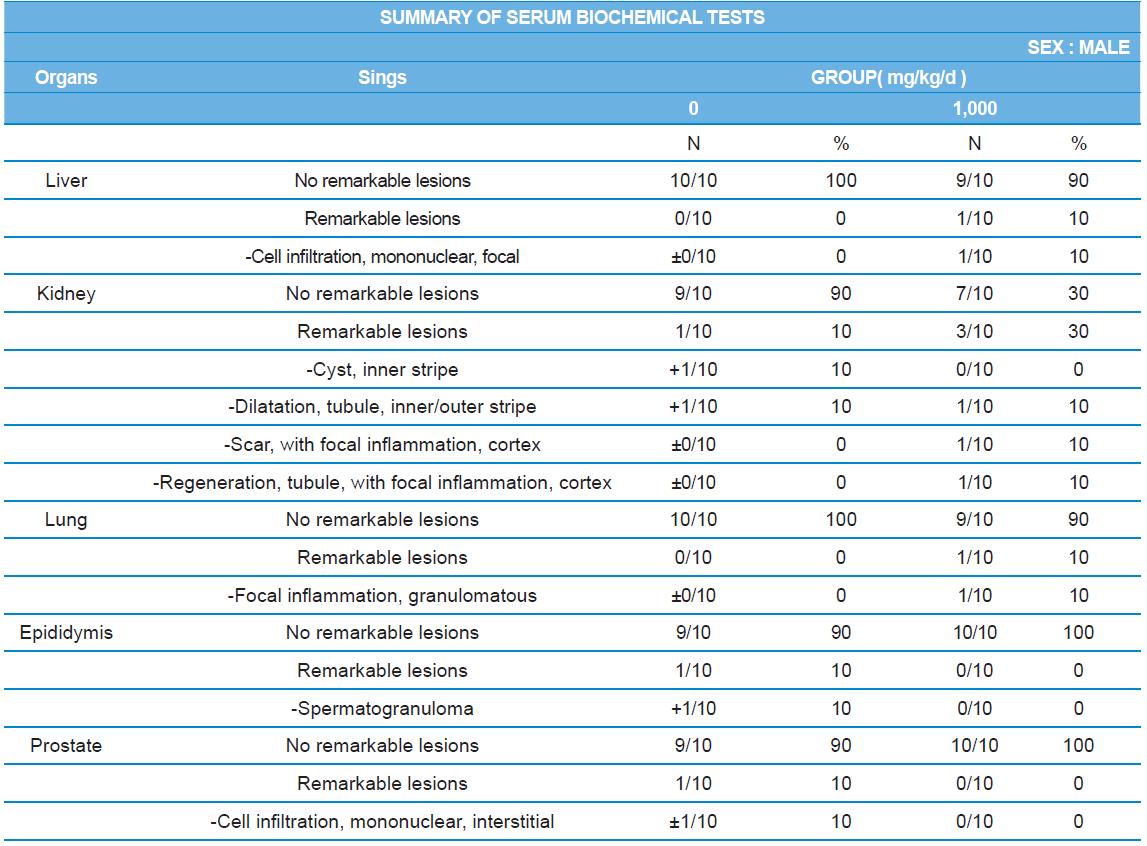
During histopathological examination of test and vehicle control groups in each sex (Tables 7-1 and 7-2), enlargement of spleen in female 500 mg/kg test group was confirmed as a congestion. However, it is not considered as a toxicological meaning because it does not show dose-dependency and it is a sporadic lesion. In male and female 1,000 mg/kg test and vehicle control group, focal mononuclear cell infiltration in liver, cyst in inner strip of kidney, tubule dilatation in inner and outer strip of kidney, scar with focal inflammation and tubular regeneration in kidney, ectopic thymus, ultimobranchial cyst, focal granulomatous inflammation in lung, spermatogranuloma in epididymis, mononuclear cell infiltration in prostate stroma were detected in both vehicle and control and 1,000 mg/kg test groups. However, it is considered not related to cricket ethanol extract administration as their severities were minimal to mild and they were present in vehicle control group as well. There was no difference in the incidence rate compared to the vehicle control group. Other than these findings, there were no abnormalities detected in the other organs.
In conclusion, under the study condition, there were not found target organs and systemic toxicological effects related with cricket ethanol extract in 4 wk oral repeated dose toxicity study. NOAEL of cricket ethanol extract administrated through oral gavage for 28 d in SD rats was found to be greater than 1,000 mg/kg body weight and acceptable daily intake is up to 10 mg per kg of body weight.
People worldwide mostly in Africa, Asia, and Latin America have been traditionally consuming about 2,000 species of insects (Premalatha et al., 2011). In current, edible insect products have been produced in North American or European countries (Hamerman, 2015). In 2015, KMFDS approved crickets as temporary food ingredient (no. 2015-09). Also, it is the one of seven eatable insect species including beetles, mealworms and protaetia brevitarsis larvae. Especially, crickets were widely consumed as not only a feeding a pet or zoo animals, but also a human food (Ahn et al., 2000). With respect to health, the environment, and livelihood, the cricket farming is likely to have advantages over the livestock business in the future (Oonincx et al., 2010). Cricket rearing requires fewer rearing coast than cattle rearing, with fewer animal welfare issues, and also poses a low risk of transmitting zoonotic infections (van Huis et al., 2013). Due to this resource use efficiency, cricket rearing for entomophagy has the potential to become a modern and sustainable food production system.
General contents of crickets have a very different trend compared to existing traditional food (Adebowale et al., 2005). Although fat and protein content showed the characteristics of animal products which are relatively large portion of cricket contents as well, it is also high in polyunsaturated fatty acid, mineral, and fiber at the same time. When assessing the value in food science, this implies that cricket has a high development potential as a functional food. Chitin is an insoluble carbohydrate which is a main component constituting the outer shell of crickets. Because chitin has an effect of enhancing immunity, crickets can be used as a dietary supplement (Wang et al., 2004). Therefore, the cricket is expected higher utilization of chitin derivatives in health supplement research in present. Cricket is high in lysine, leucine, valine, and isoleucine. These are essential amino acids which is consumed with food because it does not synthesize enough or not synthesized in human body (Belluco et al., 2013). In addition, it seems to be used for strengthening and development muscle of athletes, because the level of branched chain amino acids such as leucine, valine, and isoleucine is high in cricket (Ismasyahir et al., 2012). Crickets also contain high concentrations of fatty acids especially unsaturated fatty acids (68.6 %) such as linoleic and oleic acid (Wang et al., 2004; Kim and Jung, 2013). Unsaturated fatty acid is known as functioning of lowering blood cholesterol level (Sampath and Ntambi, 2005). Since there is relationship between a high fat content of crickets, crickets in particular contain high content of vitamin D and E (Finke, 2002). Vitamin D is recommended to consume with high Ca content food due to absorption of calcium from the stomach and functioning of calcium in the body (Ross, 2001). Vitamin E plays a key role in antioxidant (Jiang, 2014). In this aspect, it is highly advantageous to use cricket as a food source.
Recent studies have supported that crickets can be used for medicinal food and health supplement. Ahn et al. reported that G. bimaculatus extract containing glycosaminoglycan had a therapeutic potential for inflammatory disease, chronic arthritis in rats (Ahn et al., 2014). In 2015, Ahn et al. reported that cricket extract was potentially effective in anti-aging while reducing creatinine phosphokinase level in blood serum of aged rats (Ahn et al., 2015a). In addition, cricket ethanol extract inhibited adipose tissue accumulation in high phosphate dieted Wister rats. When high fat dieted rats were administered cricket ethanol extract for 1 month, fasin-related fatty acid synthesis and adipose differentiation related protein were upregulated. It is indicated that cricket can be used as an anti-atherosclerosis or inflammation medicine (Ahn et al., 2015b).
To enhance the utilization of cricket as a food material, quality improvement is being made in accordance with the various processing methods (FAO, 2011). In recent study, Ahn et al. reported the quality characteristics such as the microbial and nutrient content of crickets under different processing conditions (Kim et al., 2015). They aimed development of functional food or functional product by extracting a specific component out and increasing food quality through optimizing processing of cricket. Previously, safety evaluation of cricket powder in phosphate-buffered saline was conducted. NOAEL of cricket powder dissolved in PBS was higher than 5,000 mg/kg/d in rats of 13 wk oral dose toxicity study and there was no mutagenic effect in genotoxic evaluation(Ahn et al., 2005; Ahn et al., 2011). According to the increased interest in the edible insect, quality change in processing method of insects was emphasized. Safety evaluation of the insects under different processing method should be followed to tighten the food safety regulation. There is a need to evaluate the bio-accessibility of the nutrients and the safety of bioactive compounds with regard to human consumption.