굴 패각은 한국 남쪽의 해안의 바다 양식 폐기물로써 처리문제로 대두되고 있다. 폐기물인 굴 패각을 실용화하기 위해서, 현지 회사에서 구입한 소성된 굴 패각(COS)에 AITC (allyl isothiocyanate)를 흡착시킨 후 식품 감염 질병을 일으키는 박테리아에 대해 성장억제능력을 시험하였다. COS 분말은
Shellfish cultivation is an expanding economic activity worldwide as well as one of the major industrial sectors in coastal cities in South Korea. It is the second most important group of marine aquaculture products in Korea accounting 391060 metric tons in 2006[1]. Oyster is considered as the most common shellfish that contributes 71% of total shellfish produced in Korea[2]. However, intensive shellfish production generates a large amount of waste that accounts for hundreds of thousands of tons a year[3].
At present, in oyster shell-harvesting districts in Korea, large amounts of shells are piled up near the seaside, which creates several serious problems such as the emission of offensive odors and soil pollution from heavy metals contained in the viscera, considered as an environmental hazard. Therefore, recycling of waste oyster shell has arisen as an imminent issue. The ideal solution would be to convert the waste oyster shells to a product that is both beneficial and economically viable, and eliminates environmental problem. Oyster produces 60% waste materials of its total weight depending on different collection seasons. Several scientists tried to explore alternative uses of this waste material. Some authors reported the use of oyster shell waste as a substituent for aggregates in construction materials[4-6] or cement clinker[7]. Kwon et al.[3] applied oyster shell materials to solve problems of water eutrophication by removing phosphate from waste water.
The main component of oyster shell is calcium carbonate (CaCO3) that is converted to calcium oxide (CaO) by heat treatment, which exhibits antibacterial activity[3]. In fact, there are several reports that shell powder heated to over 700 ℃ exhibited bactericidal activity[8,9]. Oyster shell powder was also found to be applied in preparing noodles, fried chicken, sardine ball [10] and kimchi[11] for quality improvement or extension of shelf life.
In recent years, inorganic antimicrobial agents have attracted great interests for controlling of micro-organisms[12]. Sawai and Yoshikawa[13] studied the antifungal activity of metallic oxide powders (MgO, CaO and ZnO) against
Allyl isothiocyanate (AITC) is one of the most common isothiocyanates that are found in cruciferous plants either in the free form or as glucosinolates. AITC from natural sources is permitted for use as a food preservative in Japan, and as a GRAS flavoring agent in the US[14]. In addition to these applications, it was found that AITC was applied in the treatment of human prostate cancers since it acts as a cancer chemopreventive[15]. Although several studies reported that AITC was effective against various foodborne pathogens such as
AITC cannot be used along in food system as an antimicrobial compound due to its’ high volatile nature. Raw oyster shells are expected to produce porous materials at high temperature. The porous materials will allow adsorbing volatile AITC in its porous structures. The COS will be used as a carrier for the controlled release of AITC from the COS when it will be applied as antimicrobial agent. The synergistic effect of AITC and calcined oyster shell will affect on bacteria. Therefore, the aim of the present study was to investigate the antibacterial activity of calcined oyster shell (COS) and AITC adsorbed calcined oyster shell (ACOS).
COS was collected from a Korean local company, A-sung Fine Chemistry, Ulsan, Korea. AITC (>93% GC purity) were purchased from Sigma-Aldrich, Germany (Cat. no. W203410). All other reagents used were of analytical grade.
2.2. Adsorption of AITC in calcined oyster shell
COS loading with AITC was achieved via vapor adsorption by placing calcined shell in a long glass-made column (length 21 cm, diameter 2.8 cm) which was set on a AITC containing round flask. In this experiment, 4 gram of COS was taken in the column. One mL of AITC was taken in the flask. The flask was heated at 40 ℃ on a water bath. The opening of the column was sealed with aluminum foil with a tiny pore. The amount of AITC adsorbed by the calcined shell was determined by monitoring sample weight increase with time.
2.3. Characterization of particle
The particle was characterized by scanning electron microscopy and particle size analyzer.
2.3.1. Scanning electron microscopy (SEM)
An SEM equipped with energy dispersive X-ray microanalysis (Cat: JSM-6490LV, JEOL Ltd., Japan) was used to image the physical features of the oyster shell. Dried sample of oyster powder was attached to double-sided tape on the sample stubs, and then coated via gold sputter under reduced pressure. Image was obtained with an electron accelerating voltage of 10 kV.
2.3.2. Particle size analysis (PSA)
The size distributions of the COS were measured by particle size analyzer (LS 13320, Beckman Coulier, USA). A size distribution curve was plotted from the analysis.
2.4. Fourier-transform infrared measurements (FTIR)
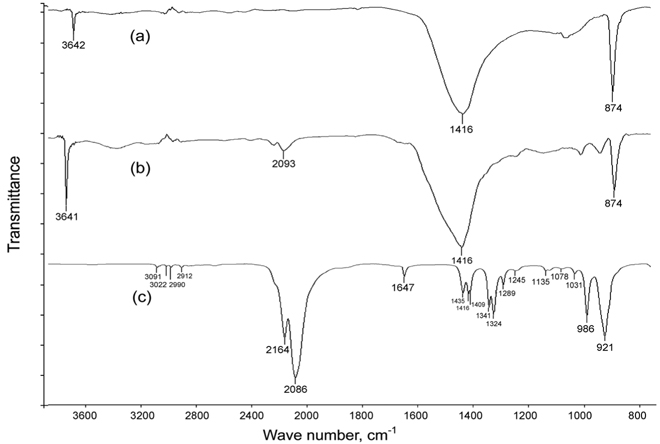
FTIR measurements were made to detect any chemical interactions between AITC and calcined oyster shell. A series of transmission spectra were obtained as: (a) calcined oyster shell, (b) calcined oyster shell loaded with AITC via vapour adsorption, (c) standard AITC. The spectrometer used was a Jasco V-670 (Jasco International Co. Ltd., Tokyo, Japan); spectra were measured over the range of 3,600-800 cm-1, with a resolution of 4 cm-1.
The test were performed against one Gram-positive and two Gram-negative food borne bacteria. Gram-positive:
From a pure 20-24 hour bacterial culture, 4-5 isolated colonies were taken and sub-cultured to a tube with 3 mL tryptone soya broth. It was incubated at 35 ℃ in a shaking incubator until it reaches or exceeds the turbidity of 0.5 MacFarland standard (corresponds to approximately 1.5 × 108 CFU/mL). The inoculum was standardized based on optical density (OD600 of 0.08-0.1) using a spectrophotometer (UV mini-1240, Shimadzu, Kyoto, Japan). Within 15 minutes of adjusting the inoculum to the 0.5 McFarland turbidity standards, it was diluted so that the final concentration achieves at 1.5 × 108 CFU/mL.
2.7. Determination of MIC of AITC
The minimal inhibitory concentration (MIC) of an antimicrobial agent is the lowest (i.e. minimal) concentration of the antimicrobial agent that inhibits a given bacterial isolate from multiplying and producing visible growth in the test system. Broth macro-dilution method was used for testing MIC. Different serial dilutions of standards AITC was prepared of which 1 mL of each dilution was added to each tube. Within 15 minutes after the inoculum was standardized, as described previously, 1 mL of the adjusted inoculum was added to each tube containing the antimicrobial agent. Each tube was mixed and incubated at 35 ℃ for 20 hours in an incubator. The MIC was determined as the lowest concentration of antimicrobial agent that completely inhibits growth of the organism in the tubes as detected by the unaided eye.
The antibacterial activity was evaluated by broth macro-dilution method counting CFU/mL. Different dilutions of calcined shell and AITC adsorbed calcined shell were prepared by mixing with sterile water. One mL of each dilution was added to each tube. Within 15 minutes after the inoculum was standardized, as described previously, 1 mL of the adjusted inoculum was added to each tube containing the antimicrobial agent. Each tube was mixed and incubated at 35 ℃ for 20 hours in a shaking incubator. After incubation, colony counting method was applied to know the number of viable bacteria. For that, 10 μL was poured on agar plate from each serial dilution. The plates were incubated at 35 ℃ for 16 to 20 hours. Colony forming unit (CFU/mL) was calculated by counting number of colony on agar plates.
All tests and analyses were repeated at least three times. The results are expressed as means±SD. A one way analysis of variance (ANOVA) and Duncan test were used for multiple comparisons using the SPSS program (IBM SPSS Statistics 20). Values were considered to differ significantly if the
3.1. Adsorption of AITC in calcined oyster shell
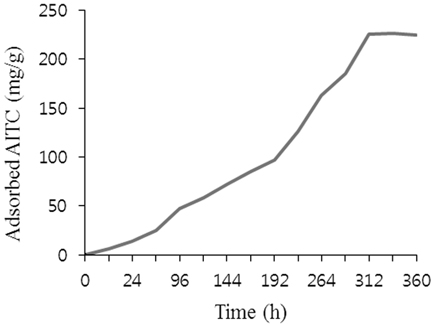
Adsorption of AITC by COS was increased with time (Figure 1). After reaching saturation level, adsorbing 225 mg/g after 312 h, it continued stable. Calcined oyster shell adsorbed AITC vapor, which indicates porous material was created upon heat treatment in oyster shell.
3.2. Characterization of particle
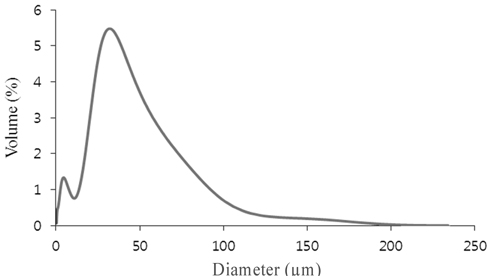
From the result of SEM, we found that the surface of the calcined oyster was highly convoluted (Figure 2). The rough and convoluted surface indicates good decomposition during calcination[3]. A distribution curve was obtained from the PSA data (Figure 3). The particle was found very fine consisting 32 μm mean size, suggesting wide surface area.
3.3. Fourier-transform infrared measurements
The presence of AITC on the calcined oyster shell powder was confirmed by FTIR. The IR spectrum shown in Figure 4 (a) represents calcined oyster shell, while spectrum (b) represents the calcined oyster shell filled with AITC. The absorbance bands at 2,164 and 2,086 cm-1 were attributed to stretching vibrations of isothiocyanate (– N = C = S) and the bands at 986 and 921 cm-1 indicated AITC stretching frequencies from (= C – CH, – CH = CH2)[17,21,22]. These bands are also shown in the spectrum (b) confirming the presence of AITC in calcined oyster shell. The subtle shifts in the AITC peaks to lower energy in the adsorbed phase confirm liquid-like structure within the adsorbed phase in the pore network.
3.4. Minimum Inhibitory Concentration (MIC) of AITC
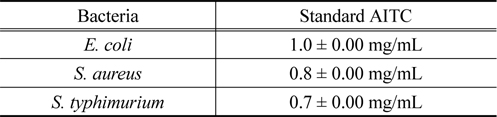
The lowest (i.e. minimal) concentration of the antimicrobial agent that inhibits a given bacterial isolate from multiplying and producing visible growth in the test system was referred to as minimal inhibitory concentration (MIC). According to MIC test,
[Table 1.] Minimum Inhibitory Concentration (MIC) of standard AITC for different bacteria
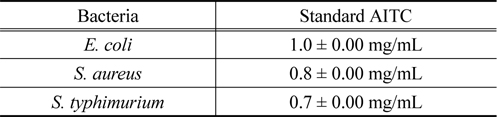
Minimum Inhibitory Concentration (MIC) of standard AITC for different bacteria
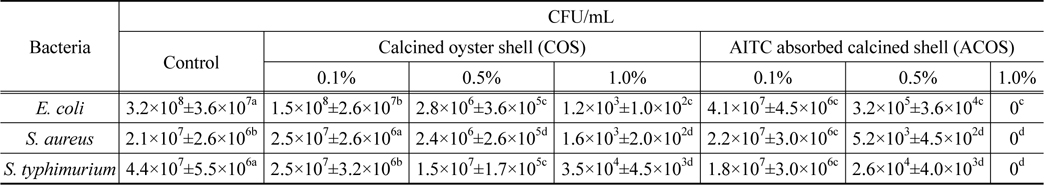
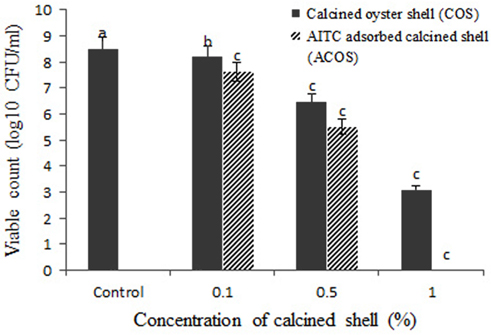
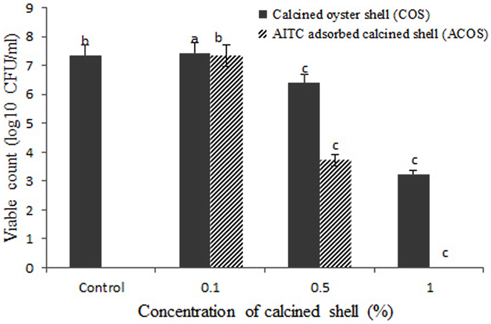
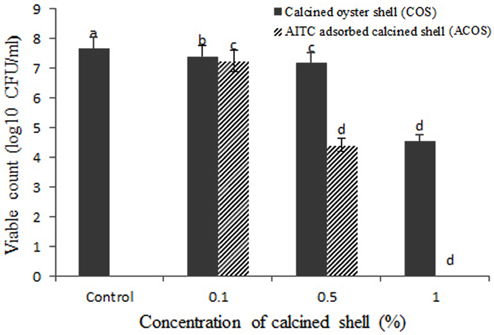
The antibacterial activity of calcined oyster shell (COS) and AITC adsorbed calcined oyster shell (ACOS) were found by estimating colony forming unit (CFU/mL). The results were shown in Table 2, Figure 5-7.
[Table 2.] Antibacterial test of calcined oyster shell and AITC absorbed calcined oyster shell
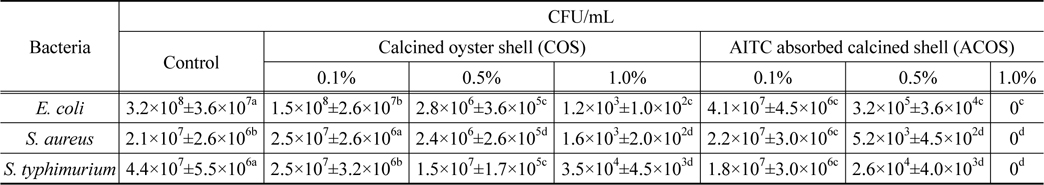
Antibacterial test of calcined oyster shell and AITC absorbed calcined oyster shell
AITC has been reported to have strong antimicrobial activity against several food poisoning bacteria such as
To reclaim large amount of waste materials produced from oyster and to explore a new antimicrobial material, calcined oyster shell loaded with AITC was evaluated. The present study has demonstrated that a waste product-oyster shells-can be transformed into an effective antibacterial material. AITC adsorbed by the calcined oyster shell enhanced the antibacterial activity against three food borne bacteria. The antibacterial activity of oyster shell was involved with the calcium oxide that was converted from calcium carbonate by heat treatment. AITC adsorbed by the calcined shell enhanced the antibacterial activity of the powder. Porous material was created by heat treatment of oyster shell. The prepared material could be explored as antimicrobial agent applied in food and other industries that facilitate the alternative use of waste produced from oyster shell.
ACOSAITC Adsorbed Calcined Oyster Shell AITCAllyl Isothiocyanate ATCCAmerican Type Culture Collection CFUColony Forming Unit COSCalcined Oyster Shell FTIRFourier-transform Infrared Measurements GRASGenerally Regarded as Safe KCCMKorean Culture Center of Micro-organisms MICMinimum Inhibitory Concentration PSAParticle Size Analysis SEMScanning Electron Microscopy