기술이전과 사업화는 매우 어려운 과제이며 국내 기술혁신 부문의 해묵은 과제 중 하나이다.특히 기술이전의 주체가 공공기관일 경우 더욱 성과가 저조한데 이는 개발기술 자체의 특성 보다는 개발자의 의식, 기술이전체제, 기술도입자의 기술수용능력, 기술사업화여건 등 여러 가지 요인에 기인한다. 본 연구는 사례분석을 통해 기술사업화 영향요인들을 구체적으로 살펴보았다. 사례분석대상과제는 국내 대표적 정부출연연구기관인 한국생명공학연구원의 초소형바이오칩 분석시스템개발기술로 하였다.
심층면접조사를 통한 사례분석 결과 기술의 시장화가능성, 개발자의 사업화 의지, 기술수용자의 보완자산 및 기술흡수능력, 양자간의 목표부합성 등이 중요한 요인임을 알 수 있다.
It goes without saying that national scientific and technological advance is a driving force to gain competitive advantage, which leads to economic progress. Successful technology commercialization maximizing the value of R&D performance is vital for survival in today’s competitive market. Public research institutions are supposed to affect long-term growth and productivity of industry through mainly licensing of the R&D performances to the firms. Despite of the effort of Korean government to promote technology transfer (hereafter ‘TT’) and commercialization of public research performances, the actual status of TT is not so satisfactory.
In this context, this study investigates a case of successful TT by a Korean biotechnology research institute to a private company. The aim of this study is to draw the factors of successful and rapid TT. The technology transferred is ‘ultra-SPR bio-chip analysis system. Based on the results of the case study, this paper draws several implications to enhance TT for the researchers and policy makers. This paper begins with a review of the relevant literature on the determinants of TT. The next section portrays the cases while the third section interprets the case describing the real role of determinants in TT. Here, research institute company will be introduced as a catalyst for TT and commercialization. In the final section, we make conclusions and implications.
2.1 Technology transfer(TT) and commercialization
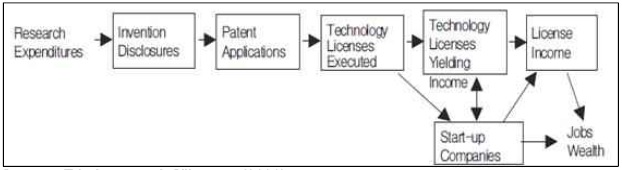
Roessner(2000) defined TT as a process of passing down of know-how, knowledge or technology from one institute to another one or private company. In large corporations, TT may occur from R&D division to production division. Traditionally there are several types of TT including, cooperative R&D, licensing or sale of intellectual property, technical assistance and information exchange. TT plays a role in virtually every instance of technology development and commercialization. In nearly every case, scientists and engineers rely on TT. They draw on knowledge developed by other that they obtain through some combination of text. Commercialization is the process of transforming new technologies into commercially successful products. It compasses a diverse array of important technical, business, and financial processes that together aim to transform a new technology into a profitable product or service(Reamer et al. 2003).Meanwhile, Friedman and Silberman(2003) define transfer of public technology (TPT) as the invention or IPR developed in public research institutes is transferred or licensed to profit organizations for commercialization(figure 1).
It is for granted that the promotion of TT and commercialization is a very important component for economic development. However, for several reasons, TT has not been conducted in a successful way, either by public or private organization. The way how TT facilitates effective technology development and commercialization is a major question.
2.2 Determinants of technology transfer
TT is a complex of simultaneous processes and has various determinants. Rahal and Rabelo(2006) identified the determinants and decision factors that influence or impact the licensing and commercialization of public technologies. Rahal and Rabelo(2006) classifies technology transfer determinants as six major types : institutional determinants, inventor-related determinants, technology-related determinants, market and commercialization-related determinants and intellectual property-related determinants. Our study was conducted according to that categorization and therefore we are going to describe the theoretical review on those factors in more detail. Institutional determinants are classified as technology transfer office(TTO) determinants(Hauksson, 1998a; Hauksson, 1998b), universities licensing policies determinants(Hsu & Bernstein, 1997) and institutional prestige influence determinants(Sine, Shane, & Gregorio, 2003). Inventor-related determinants are classified as inventor involvement and cooperation as a team player(Jensen & Thursby, 2001; Thursby & Thursby, 2002) inventor being recognised as a technology leader(Allen, 1977; Berry, & Broadbent, 1987; Berry, & Broadbent, 1984), inventor credibility in the field(Allen, 1977; Berry, & Broadbent, 1987; D.C. Berry & Broadbent, 1984), inventor has realistic expectations about his or her technology(Galbraith, 1990), incentives to inventor by the licensor(Jensen & Thursby, 2001). Technology-related determinants are technology nature and sophistication, technology’s significant benefits and advantages technology’s quantifiable benefits and advantages the technology’s degree of compatibility to other necessary technology(Rogers, 1995). Commercialization-related determinants are classified as the technology’s identifiable current and immediate market needs, the absence of a dominant competitor in the technological market size, the technology’s market growth anticipation, technology’s expected market trend the time for the technology to reach the target market penetration, market accessibility for the technology. Intellectual property-related determinants are classified as the complete and clean technology’s literature search, the completed patent search, and the patent search is clear and clean, the confidentiality of the technology (no oral or written disclosures), the technology has no prior claims, the strength of intellectual property, exclusivity of intellectual property, exclusivity of intellectual property(Rahal & Rabelo, 2006).
TT process typically composes of characteristic of technology, agent, transfer medium, transfer recipient(Bozeman, 2000).
2.2.1 Characteristics of technology providers
Technology provider is often developer of the technology such as research institutions, inventors. Many of the previous empirical studies related to technology transfer point the developer's qualifications, or dedication of an organization for technology transfer as main factors of performance of TT. The performance of TT may depend on the nature of R&D institutes. Even ifit is the same, different technology transfer strategies are needed by the recipients of technology. This type of technology transfer determinants is inventor-related determinants. They are classified as inventor involvement and cooperation as a team player(Jensen & Thursby, 2001; Thursby & Thursby, 2002), inventor being recognised as a technology leader(Allen, 1977; Berry, & Broadbent, 1987; D. C. Berry & Broadbent, 1984), inventor credibility in the field(Allen, 1977; Berry, & Broadbent, 1987; D. C. Berry & Broadbent, 1984), inventor has realistic expectations about his or her technology(Galbraith, 1990), incentives to inventor by the licensor(Jensen & Thursby, 2001).
2.2.2 Characteristics of technology
The technology itself is the subject of transfer and may be considered as one of the most representative determinants of technology transfer performance. Jensen and Thursby(2001) surveyed technology transfer data in 62 universities of the United States. According to them, the inventions at the universities are mainly early staged and therefore it is difficult to find the companies which were interested in those technologies. Many companies would not be positive to invest a lot of money and time to commercialization of immature technology.
This phenomenon is similar in the results of domestic research. Korea Industrial Technology Association conducted a research, in the information and communication industry, on the respective commercialization degree of software, systems and finished products, information and communications service, parts and components. They found that among them the commercialization of software, systems and finished products are the highest. These results may be caused by technological uncertainties. According to the study of Goel et al.(1991), the higher technological uncertainty, the useful the contracting R&D or technology collaboration in order to lower the uncertainty byadditional resources.
Huang et al.(2010) suggest that technology it self is the most important determinant. According to them, technology determinants are technology nature and sophistication, technology’s significant benefits and advantages as identified and perceived by the user, when compared to current competing products, technology’s sustainable competitive advantages and superiority as perceived by the user, the availability of a functioning prototype, the technology’s degree of compatibility to other necessary technologies(Rogers, 1995), technology scope or future uses, technology uniqueness and superiority, etc.
2.2.3 Recipients of technology
The characteristics of technology recipients can be represented as complementary assets related to production, sale, distribution except technology , and absorptive capacity of technology. The importance of absorption capacity of the technologies are claimed by many researchers. Cohen, W. & Levinthal, D.(1990) claim the ability to absorb the imported technology from a third party is necessary condition of the successful commercialization. Sher et al.(1997), in their study on international technology transfer, the absorption capacity of the company in Taiwan, affect the efficiency of international transfer of technology. In addition, Lin et al.(2004), defined the company's ability to absorb technology asthe keyfactor affecting transfer performance. Mangematin and Nesta(1999) also found in their study that when absorption capacity of companies is poor, effectiveness of technology licensing or collaborative research falls. Eventually, the companies that make their technology transfer successful have adoption capability which represents the technology capacity as well as complementary ability which representscapital assets.
2.2.4 Technology intermediaries
The third type of technology transfer determinants is technology intermediaries. They transmit technologies from the lab to industry and are called “institutional determinants”. They are classified as technology transfer office(TTO) or technology licensing office(TLO) or transfer agents(Hauksson, 1998a; Hauksson, 1998b), universities licensing policies determinants(Hsu & Bernstein, 1997) and institutional prestige influence determinants(Sine, Shane, & Gregorio, 2003). In the aspect of TTO determinants, Tornatzky(2000) argued appropriate staffing, clearly articulated mission, customer-friendly orientation, clear policies and procedures, supportive university culture are vital for TTO practice.In the United States, where technology transfer is the most active, technology transfer and commercialization policy represent the collaboration program among industry university and research institute. Examples of the organizations in charge of these programs are NASA's National Technology Transfer Center(NTTC), and Regional Technology Transfer Center(RTTC), and the Federal Institute Consortium(FLC).
2.2.5 Government TT Policy and Environment
Technology transfer is influenced not only by the characteristics of the technology itself, technology providers and technology intermediaries andadopters but also determined by the relevant government policies and environmental factors. The performance of TT, such as license contract or royalty income is dependent on the spillovers of the technologies diffused to industry. The diffusion of technologies is promoted when the access to the infra of TT including lawyers, venture capitalists, consultants, entrepreneurs and developers is easy.
Government policies and environment related to TT aredetermined considering the characteristics of each country. In addition, to make TT more successful, the determinants of TT such as characteristics of technology, technology provider, intermediary, recipient and policy and environment related to TT are to be organically connected to each other.
In the following sections we are going to examines the process of TT as well as the characteristics of the factors in the case study and analyze thedeterminants of successful TT.
3.1 Technology providers: Korea Research Institute of Bioscience and Biotechnology(KRIBB)
3.1.1 General information
Korea Research Institute of Bioscience and Biotechnology( hereafter ‘KRIBB’) was established in February 1985 as a government-funded research institute. The mission of KRIBB is to carry out R&D activities and related projects in the field of bioscience and biotechnology in joint effort with other research institutes, academic, and businesses at home and abroad and Another mission of KRIBB is to disseminate the results of its scientific research and technological development.
The vision of KRIBB is to become a global research institute leading Bio-Innovation for the humankind(fig.2).
Total human resource is 1,125 including 339 regular employees. KRIBB has 5 research divisions such as Biomedical Genomics Research Center, Biomedical Protemics Reseach Center, Aging Reseach Center, Bio Nano Technology Reseach Center, and Bio Medical Translational Research Center
3.1.2 Technology Transfer Activities
KRIBB has a technology transfer office(TTO) to bridge the gap between innovation and the Real World Applications. The business development based on the technologies of KRIBB has been done by the Department of Intellectual Property Management which is playing a role as a technology transfer office(TTO). The idea or know-how as well as the technologies developed form the R&D centers are detected by the Technology Evaluation Committee of KRIBB run by the TTO of KRIBB, and their market and business values for creating new bio industry are also assessed. The selected technologies are actively licensed out to market leaders including domestic and global companies. Nurturing and incubating start-ups are another important function of the TTO of KRIBB. The joint venture with established partner company could be created by providing with highly valued technology.
Main function of TTO in KRIBB is knowledge(intellectual) property management, technology valuation, marketing, negotiation for transfer, technology licensing-out and business incubation. The activities compose of consulting of IP(Intellectual property), screening of idea, arranging fund, investment for spin-off KRIBB companies and incubating bio-tech, start-ups at Bio-Venture Center(BVC)
3.1.3 The technology developed and transferred : the world’s smallest biochip analysis system developed
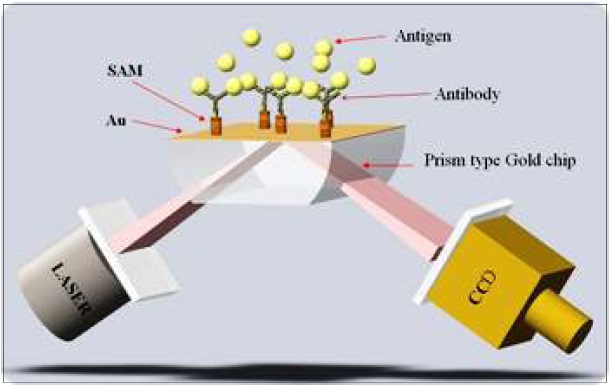
On December 24, 2008 that a research team led by Dr. Bong Hyun Chung and Dr. Yong-Beom Shin at the BioNanotechnology Research Center of KRIBB succeeded in developing the world’s smallest SPR biochip analysis system(fig 3). Surface Plasmon Resonance (SPR) refers to a phenomenon of light absorption caused by free electron clouds on the surface of a very thin layer of gold or silver. The research team utilized this phenomenon to analyze the amount of adsorbed molecules to the surface of thin metal layer without using any label such as fluorescence.
The original idea for this minimizing technique was derived from the generation of infinite diverging emission of oval shape by incidence of laser to the middle of symmetric mirrors rotating at a speed of over 1500rpm/min. The minimization was enabled by adopting a short ray path to the system so that interference could be removed while still permitting use of the laser.
It was expected that the results of this success will be applied to a wide range of areas including the development of methods of diagnosing diseases, research into the development of new drugs by using the interaction between physiological molecules, the development of defensive measures against bio-terror for military purposes, the evaluation of food safety through the detection of residual agrichemicals in agricultural and marine products, and the evaluation of contamination in water sources.
The excitation of Surface Plasmon by light is denoted as a Surface Plasmon Resonance(SPR) for planar surfaces or Localized Surface Plasmon Resonance(LSPR) for nanometer-sized metallic structures. This phenomenon is the basis of many standard tools for measuring adsorption of material onto planar metal (typically gold and silver) surface or onto the surface of metal Nanoparticles. It is behind many color based biosensor applications and different lab-on-a-chip sensors.
The operating principle of SPR is as follows. It use laser diode as light source and use rotating mirror (KRIBB has patent) as polarized light. Incoming beam interacts with the plasma waves on the gold surface and excites the Plasmon and CCD image sensor detects reflected light, which is excited Plasmon from the gold surface.
One of the features is that SPR reduces an interference phenomenon using short or straight wave and Maximize beam power uniformity of two dimension laser. Another feature is high applicability an properties analysis for chemical and Bio materials, which is absorbed an sensor chip. These features make high degree of precision, which is the main concern of small SPR sensor system. As for the patent, manufacturing smallest SPR biosensor system has technology patent for miniaturize hardware (SPR using rotating mirror) at home and abroad.
3.2 Technology recipient : KoMiCo
KoMiCo was founded in February of 1996, and developed cleaning and refurbishment technology for cleaning the high-cost parts that go into the devices that make semiconductors and LCDs. Starting with the founding of R&D center in Aprill of 1998, it expanded form cleaning etch device parts to CVD, sputter and all of the semiconductor manufacturing processes. It also expanded into supporting manufacturers of other kinds of displays kike PDPs and OLEDs. IT also built a new, state-of-the-art facility in 2001. This has improved our work environment, product quality, turn-around-time, and responsiveness to customer needs, allowing the Korean semiconductor and LCD industries to reduce costs and increases yields. Since then, it have now grown beyond the Korean market to become a global precision cleaning company.
In December of 2001, KoMiCo announced its determination to become the leading precision cleaning company by registering with KOSDAQ. Using accumulated technologies in the cleaning business, we expanded into the refurbishment and spare parts businesses. In July 2005, it founded MiCo C&C in Cheonan, Korea to specialize in refurbishment and part manufacturing for the display industry.
KoMiCo harnessed the advanced ceramics technology to develop precision MLC parts and other critical component manufacturing to further satisfy their customer’s needs. Main business area of KoMico is SOFC, precision cleaning, material technology, special coating, MLC technology and manufacturing.
3.2.1 Process of TT
As described earlier, the process of TT in KRIBB consists of knowledge(intellectual) property management, technology valuation, marketing, negotiation for transfer, technology licensing-out and business incubation. Meanwhile, the process of TT of the case is a little different in that the final output is set-up of a research institute company with the help of Daedok Innoplois, which is a public organization established for promotion of R&D and commercialization in Daedeok special zone.
3.2.2 Intellectual property management
At the stage of intellectual property filling and management, KRIBB utilized screening and consulting of in-house patent attornies and registered technology patent for miniaturize hardware(SPR using rotating mirror) at home and abroad.
Despite the smooth development of technology and patent registration, it was difficult for KRIBB to estimate the marketabililty with limited information to judge the future expansion of the market as is the case of most high technology. The success of commercialization of disruptive technologies is difficult to determine as there is no existing markets. However, considering the exellency of R&D accomplishment, KRIBB finally decided to transfer the technology.
3.2.3 Consideration of marketability
Despite the smooth development of technology and patent registration, it was difficult for KRIBB to estimate the marketabililty with limited information to judge the future expansion of the market as is the case of most high technology. The success of commercialization of disruptive technologies is difficult to determine as there is no existing markets. However, considering the exellency of R&D accomplishment, KRIBB finally decided to transfer the technology.
3.2.4 Looking for a right partner
Seeking business partners with a source technology is always very difficult. KRIBB had difficulty in finding the right partner to commercialize SPR technology.A few companies have been contacted in the initial stage of seeking partners, but it turned out that most of them are lack of complementary assets or sufficient technology to commercialize.
In the course of looking for a partener, KRIBB happened to meet KoMiCo which has a good reputation in the fieldof the semiconductor and semiconductor manufacturing equipment, precision cleaning of parts, etc. KoMiCo was also pursuing a new business chance, making use of IT accumulated for many years. In fact, taking the long recession of semiconductor to the world market into account, they were looking for a new business, with a convergence technology between IT and BT.For KoMiCo, SPR biochip technology developed by KRIBB was considered to be suitable for their plan.
3.2.5 Barriers to proceed
However, two large entry barriers to market existed. The first one was about environmental factors caused by a system requirements for real-time bio-molecules using methods for detection and diagnosis. The second one was market-related. There was a risk in the market as the reputation and market share of existingproducts and reliablity of the customers was high enough. Therefore, they could not make a decision easily on this issue.
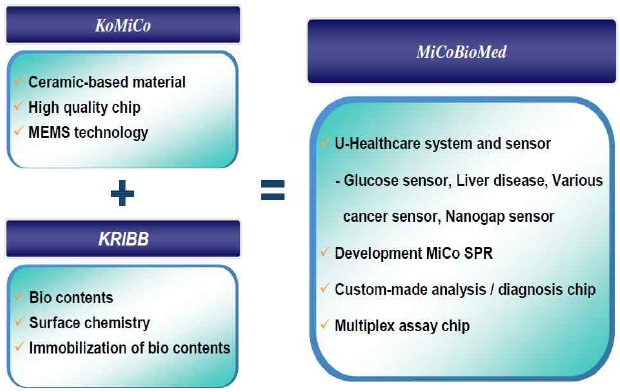
In the end, on July 7, 2009, because both parties had mutual confidence in each other’s technology power, they finally reached an contract of TT with a condition of 3 billion won royalties, resulting in a venture company called MiCoBioMed. This company is second research institute company established by KRIBB.
3.2.6 TT and commercialization
After having founded MiCoBioMed, both parties established a close system of TT and completed a successful and a rapid TT as well ascommercialization. The period of the TT is considered as the shortest in the history. So far, several products have been manufactured and sold. Within five years, sales of more than 30 billion won is expected to occur. In fact, after initial sales, they got very favorable responses from overseas, including a contract with a foreign company more than 300,000 U.S. dollars. Additional performances will be described later in detail.
3.3 Establishment of a research institute company: MiCoBioMed
MiCoBioMed was established in July 2009 as a joint venture of industry-leading IT-based company (KoMiCo) and the government-owned BT-based Korea research institute of Bioscience & Biotechnology(KRIBB). Beginning with the world's smallest SPR Bio-chip analyzing system, MicobioMed is searching for a one testing device with various capabilities, which can be of use anytime, anywhere. MicoBioMed is currently developing and expanding various applications for its SPR product.
From providing a blood sugar testing strip, which is most consumable item for diabetes testing, MiCoBioMed is aiming to provide U-health care service by developing Bio-testing products for customer based interface. Also, MiCoBioMed will aim to be a leading Bio company in the world with investment on research and human resource as well as developing new products with leading technology that can provide fast and accurate results.
3.3.1 Utilization of public support program: research institute company system
The establishment of MiCoBioMed was helped by research institute company system. The research institute company is a company launched by government-funded research institutes including public research institutes. In the case of Daedeok Innopolis, a district in Korea specifically designated to promote R&D, research institutes located within it, to sell their own technology, found such a company in the district by financing 20 percent or more of its capital. The research institute company system is a systematic way of ensuring that the research outcome of the government-funded research institutes actually be put into producing profit, and is characterized by the ability of those institutes to establish a business themselves marketing their own technology and participate in running it. Through this system, the government-funded research institutes, aside from serving as the R&D hub, expand their role to take on commercializing and promoting the use of their research outcome while the research companies take the lead in the market by integrating the superior technology of the research institutes, private-owned capital, and administrative know-hows.
The research institute company system, implemented as a means to directly translating research outcome into business, now in its fifth year, has just gained its momentum. Ever since 2005, the first year of implementation, there has been a steady increase in the number of companies being approved every year.
As mentioned earlier, technology itself that is the subject of technology transfer can be called the most representative factors of in determining the performance of TT. The patent of the smallest SPR biochip system was registered in Korea and abroad. Source technology and essential equipments had been developedprior to comercialization of this technology. In addition, by the differentiation from existing products through miniaturization, the technology is evaluated as deserving world-widely recognized. Accordingly, even prior to TT process, the likelihood of successful commercialization was recognized with just source technology.
4.2 Reputation and competence of technology provider
Technology providers can be represented as the developer's qualifications and experience and dedication of TT organization to technology transfer, the characteristics of the research institutions and association with industry.
From the perspective of developer of the technology, Dr. Bong Hyun Chung in KRIBB has conducted R&D bearing commercialization in mind since the early-stage the R&D.Dr. Chung usually does not like to take up a research project if it does not have a enough chances of application. As he always has marketability in mind from the start of the study, the value of developed technology could be highly appreciated in the market. The high amount ofroyalty-3 billion won-was possible.
The characteristics of these technology developers or provider affects a lot to commercialization of the technology. The organization, KRIBB also has a strong commitment to strengthen cooperation betweenthe researchers and biotechnology companies. The activities include launching of Connect KRIBB, Bio-technical support center, Consulting center for patenting and technology commercialization, and commercialization promotion programs such as the outsourcing of professional organizations for commercialization..etc. Thanks to such characteristics of the technology provider and developer, a successful TT could be done and they could creat such a good output as MiCoBioMed.
4.3 Characteristics of technology recipient
As described earlier, the characteristics of technology recipients in TT represent technology absoptive capacity and complementary assets such as manufacturing, sale, distribution, except technology. CoMiCo is a company with more than 10 years of history in the field of semiconductor and electronics manufacturing equipment and in the field of precision cleaning of parts. In addition, the company's complementary assets such as production, sales, distribution seem to be enough to commercialize the tiny SPR technology. Even if the company have, to some extent, the complementary assets, it should have the capability to absorb and digest the technology transferred from public research institutions. CoMiCo has an extensive experience in the IT industry, They has sufficient capacity to absorb technology and production capabilities such astechnologies needed for Compact SPR biochip analysis system and the semiconductor-related technology. In addition, the management philosophy of CEO matched the technology provider. The mix of the exellent technology of KRIBB and marketing and sales experience in technology and complementary assets of CoMiCo are the factors of successfultransfer of technology.
4.4 Utilization of government support program
In the course of TT, KRIBB made use of a public TT support program at a proper way, the name of which is INNOPOLIS. The purpose of business of INNOPOLIS is creating the national new growth power by promoting research and development of industry-academies and research institutions within the special zone and by supporting commercialization of research accomplishments and business initiations.
It was established jan 2005 by a special law for nourishing Daedeok research and development special zone. Guidelines for future business of INNOPOLIS is promoting commercialization through activation of market functions, maximizing business effects through selection and concentration’strategy and differentiated strategy only by Daedeok research and development special zone.
Ⅴ. Conclusion and Implications
There is no doubt that TT and commercialization maximize the value of research outcome. In Korea, recently government investment in R&D has sharply increasedand has tried various policies to promote TT and commercialization, but the extention of utilizing R&D performance falls far short. In addition, more than 80% of doctoral degree holders belongs to public research institutions and the majority of country total R & D budget is being invested in thepublic research organizations. To make the national wealth lead the improvement of national competitiveness, TT and commercialization of public researches is essential.
It is true that, despite the efforts of Korean government, TT and commercialization in the government funded research institutes is not so active, by lack of expertise, data base, dedicated staff and so on. In this context, the purpose of this study to investigate, through a case study, the success factors of TT and determine the factors to focus to improve technology transfer performance.
Through the analysis of this case, we could identify several determinants as follows. First, it is the degree of excellence and development of the core technology. For a successful TT and achievements, the core technology itself should have excellent marketability as well as the technological capacity. The closer the technology to the market, much more likely to succeed and commercialization of the technology. In addition, however original and excellent the concept of technology itself is, successful technology transfer is difficult to expect if commercialization is difficult or there is alack of essential equipment.
Second, it is the nature of technology providers. Researchers should have an commercialization oriented attitude in mind from the planning stage of the technology development projects. If they have a certain degree of knowledge on techno-marketing, it would be excellent.Even though technology itself is excellent and marketable, TT can not be made successful if technology suppliers, public institutions are not equipped with suitable system or active mind. Therefore, a systematic and professional TT support system and aggressive technology commercialization oriented mind is needed in the government funded research institutions.
Third, it is the nature of technology adopters. Technology recipient company should possess complementary assetsand technological absorption capacity for technology commercialization. Poor ability to absorb technological competence can lead to commercialization difficult. If the adopter does not have an active and open attitude, mutually fruitful cooperation in the process of commercialization will not be easy to expect.
Fourth, looking for suitable partners is important in TT. In order to get a successful TT, goals and objectives of the technology provider or transfer agencies and the company's business should be matched. In addition, according to the characteristics of technology and institution, TT should take appropriate form - such as collaborative research, licensing, joint ventures, andtechnology transfer. Also, to find a right partner, technology and companies should be thoroughly evaluated at the preparation steps.
In this case, we could find the factors influencing the successful TT and commercialization. KRIBB is seen to go through good process in the TT preparation with partenar as a public research institution and commitment of CoMiCo did an important role. The implications that can be drawn here is thatpublic research institutions should focus a commercialization-oriented R&D managementaway from project management-oriented one. For that they should have a techno-marketing mind from the planning stage. They should also continue to pursued to improve collaboration. In this case, a public technology transfer program of technology transfer was utilized in a proper way. The parteners shouldidentify facilitators or important linkages between public research institutions and industry to promote TT. The industry should have a clear business objectives, complementary assets and technology absorptive capacity. They are important to check prior to TT process. When these factors are well formed , more rapid and successful TT will be possible as this case and the probability of business failure is expected to be reduced.
Asresearch study is conducted as a form of case study and in one institute, it is recommended to take care of generalize the result. A more comprehensive case analysis and statistical analysis will be needed in future.