Nature plays an important role in providing the basic needs of human in the production of food-stuffs, shelters, clothing, means of transportation, fertilizers, flavours and fragrances, and, not the least, medicines for the treatment of various diseases (Cragg and Newmann, 2013; Gordaliza, 2007). Mineral, animal and plant products were utilized as the main sources of drugs, and the use of natural products with therapeutic properties is as ancient as human civilization (De Pasquale, 1984; Rates, 2001; Samuelsson, 2004). Most of the plant compounds that have been found to be medicinally useful and interesting tend to be secondary metabolites including alkaloids, phenolics, acetogenins and terpenoids. Secondary metabolites represent features that can be expressed in terms of ecological, taxonomic and biochemical differentiation and diversity. The wide chemical diversity of secondary metabolites throughout the plant kingdom represents an extremely rich biogenic resource for the discovery of novel drugs and for developing innovative remedies (Gurib-Fakim, 2006). To date, natural products and their derivatives represent more than 50% of all the drugs in clinical use in the world. Higher plants contribute no less than 25% of the total. During the last 40 years, at least a dozen potent drugs were reported from flowering plants (Gurib-Fakim, 2006).
The Thymelaeaceae family is a cosmopolitan family of flowering plants, which is established by Hanus-Fajerska et al. (2012). This family consists of 45 genera and 700 - 800 species, and is widely distributed in both hemispheres (Herber, 2002; 2003). Nine genera and 89 species of the Thymelaeaceae plants are endemic to China (Zheng et al., 1999). In the other large genera of the Thymelaeaceae are Gnidia with approximate number of species 160, Pimelea (110), Daphne (95), Wikstroemia (70), Daphnopsis (65), Struthiola (35), Lachnaea (30), Thymelaea (30), Phaleria (30), and Gonystylus (25) (Kubitzki and Bayer, 2003). The species of this family include mostly shrubs or small trees, rarely herbs, evergreen or deciduous. Most species are toxic but some have medicinal properties. The phloem contains strong fibers, which make the bark of many species beneficial in manufacturing of high quality paper especially bank notes. The stems have characteristics of supple and are difficult to break, and used as a substitute for string (Zheng et al., 1999). One of the plants within the Thymelaeceae is Phaleria macrocarpa (Scheff.) Boerl. which was first described by Scheffer as Drimyspermum macrocarpum based on fruiting specimens collected by Teysmann near Doré, in western New Guinea (Angiosperm Phylogeny Group, 2003). The other botanical name of this plant is Phaleria papuana Warb var. Wichanii (Val) Back (Hou, 1960). This plant is popular with the name of ‘Mahkota dewa’, which is literally translated as God’s Crown. It is locally known as ‘Simalakama’ in Sumatra (Malay) and Depok (West Java) and ‘Makutadewa’, ‘Makuto rajo’, ‘Makuto ratu’ or ‘Makuto mewo’ in Java (Harmanto, 2005).
P. macrocarpa is a shrub or small tree that grows throughout the year. This plant usually reaches the height of 5 m but sometimes its height could also reach up to 18 m (Harmanto, 2003; Stevens, 1974; Winarto, 2003). It grows in areas of 10 – 1,200 m above the sea level and the most productive age of this plant is in between 10 – 20 years (Saufi, 2007). The plant of P. macrocarpa has features of many-branched crown with 1-metre long straight root exuding sap, a brownish green bark and white wood. The leaves are green, sharp edge and tapering from 10 - 15 cm in length and 3 – 5 cm in wide (Fig. 1A). Its flowers appear in white colour with trumpet-like shape and produce pleasant smell. The fruits have an eclipse shape; occur in various sizes with diameter ranging from 3 – 5 cm. Its fruits have smooth surface and changing their colour from green when young into red or maroon when ripening (Fig. 1B). The pit is round, white and poisonous (Fig. 1C) (Altaf et al., 2013; Hendra et al., 2011; Saufi, 2007)
P. macrocarpa is frequently used as a therapeutic healing alternative in health system of the Indonesians and lower course of Malaysia (Ali et al., 2012). All parts of this plant including fruits, seeds, stems and leaves have well known therapeutic properties and have been extensively used in traditional medicine (Tjandrawinata et al., 2010 Winarto, 2003). Specifically, the fruits of P. macrocarpa are used to treat flu, rheumatism, heart diseases and cancer; the leaves are used to treat dysentery, allergy, tumour and impotency while the stems are beneficial in the treatment of bone cancer. The eggshells of seeds are used to counter breast cancer, cervix cancer, lung disease, liver, and heart diseases. This plant especially the seed part cannot be consumed directly due to its high toxicity which can cause swelling, numbness and unconsciousness. However, the seeds can be used as an external medicine for the treatment of skin conditions and for ornamental cultivation purposes, which act as a traditional biopesticide (De Padua et al., 1999; Harmanto, 2003).
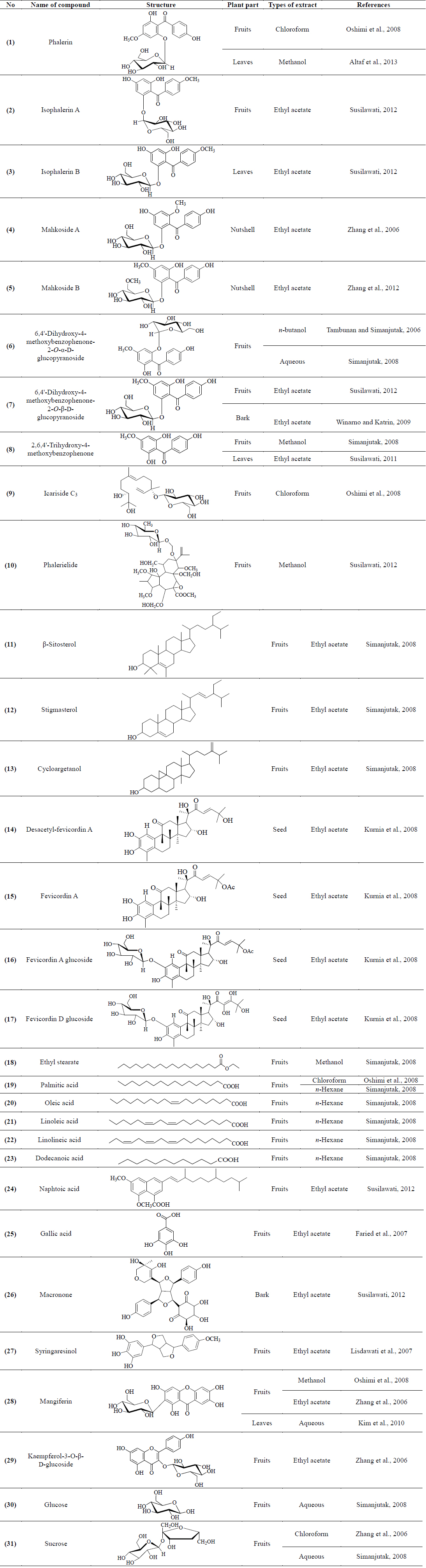
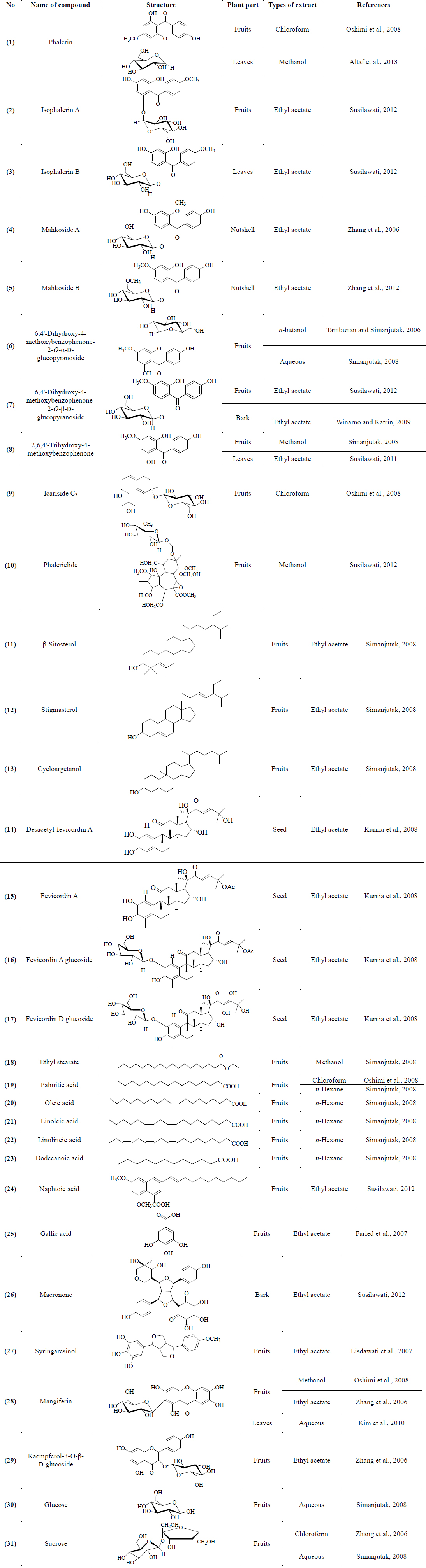
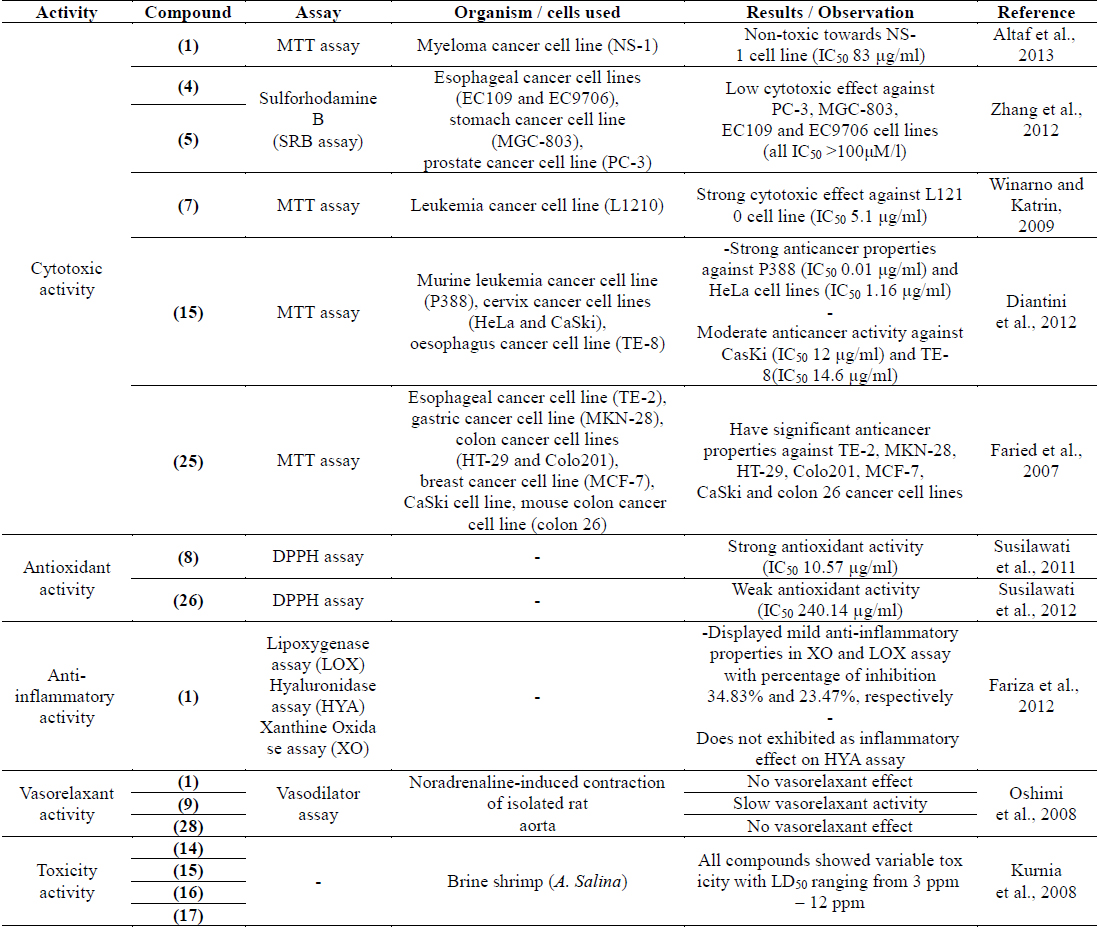
Several research groups especially from Indonesia and China had extensively carried out studies to find chemical constituents from P. macrocarpa. The studies resulted in the isolation of several classes of compounds such as benzophenones, terpenoids, xanthones, lignans, acids and sugars. Chemical investigation on the fruits, leaves and bark of P. macrocarpa afforded eight benzophenone derivatives, identified as phalerin (1) (Altaf et al., 2013; Oshimi et al., 2008), isophalerin A (2), isophalerin B (3) (Susilawati, 2012), Mahkoside A (4) (Zhang et al., 2006), Mahkoside B (5) (Zhang et al., 2012), 6,4'-dihydroxy-4-methoxybenzophenone-2-O-α-D-glucopyranoside (6) (Tambunan and Simanjutak, 2006), 6,4'-dihydroxy-4-methoxybenzophenone-2-O-β-D-glucopyranoside (7) (Susilawati, 2012; Winarno and Katrin, 2009) and 2,6,4'-trihydroxy-4-methoxybenzophenone (8) (Simanjutak, 2008; Susilawati et al., 2011). Several triterpenoids derivatives known as icariside C3 (9) (Oshimi et al., 2008), phalerielide (10) (Susilawati, 2012), β-sitosterol (11), stigmasterol (12) and cyloargetanol (13) (Simanjutak, 2008) were successfully isolated from the fruits of P. macrocarpa. Phytochemical studies on the fruits by Kurnia et al. (2008) reported a new 29-norcucurbitacin derivative named as desacetyl-fevicordin A (14), together with fevicordin A (15), fevicordin A glucoside (16) and fevicordin D glucoside (17).
In addition, studies on chemical constituents from the fruits of P. macrocarpa revealed the isolation of an ester compound, ethyl stearate (18) (Zhang, 2006) and acid derivatives including palmitic acid (19) (Simanjutak, 2008; Zhang et al., 2006), oleic acid (20), linoleic acid (21), linolineic acid (22), dodecanoic acid (23) (Simanjutak, 2008), naphtoic acid (24) (Susilawati, 2012) and gallic acid (25) (Faried et al., 2007). A novel lignan named as macronone (26) (Susilawati et al., 2012) and a known lignan, syringaresinol (27) (Lisdawati et al., 2007) were obtained from the ethyl acetate extract of the bark and mesocarp of P. macrocarpa, respectively. The investigation on the chemical constituents of this plant yielded a xanthone and flavonoid compound identified as mangiferin (28) (Kim et al., 2010; Oshimi et al., 2008; Zhang et al., 2006) and kaempferol- 3-O-β-D-glucoside (29) (Zhang et al., 2006). Moreover, two sugar molecules known as glucose (30) and sucrose (31) (Simanjutak, 2008; Zhang et al., 2006) were isolated from the aqueous extract of P. macrocarpa fruits. Furthermore, the quantitative analysis on various parts of P. macrocarpa fruits revealed the presence of five major flavonoids named as kaempferol (32), myricetin (33), quercetin (34), naringin (35), and rutin (36). Qualitative analysis of the flavonoids was carried out by reversed-phased high performance liquid chromatography (RP-HPLC) using an analytical column C18 60Å 4μm, 3.9 × 150 mm, Waters, NANPA, MA (USA). The flavonoids were detected at 365 nm of UV-Vis photodiode array (DAD) detector (Hendra et al., 2011).
Empirically, Indonesian people have as often utilized the fruits bark and leave of P. macrocarpa for the treatment of various diseases such as cancer, diabetes mellitus, allergies, kidney disorders, blood diseases, stroke and acne with satisfactory results. Therefore, many scientific evaluations on bioactivities of P. macrocarpa have been conducted in order to prove the traditional claims on the medicinal values of this plant.
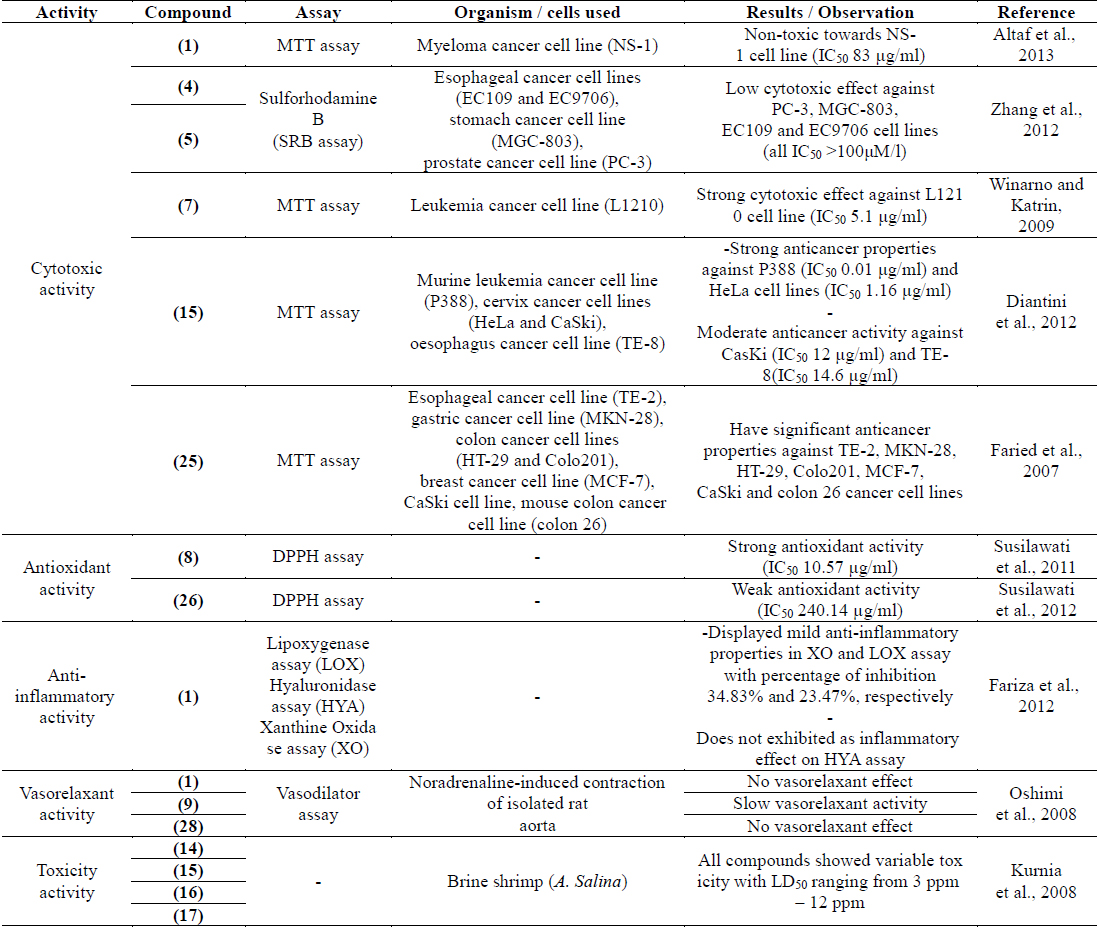
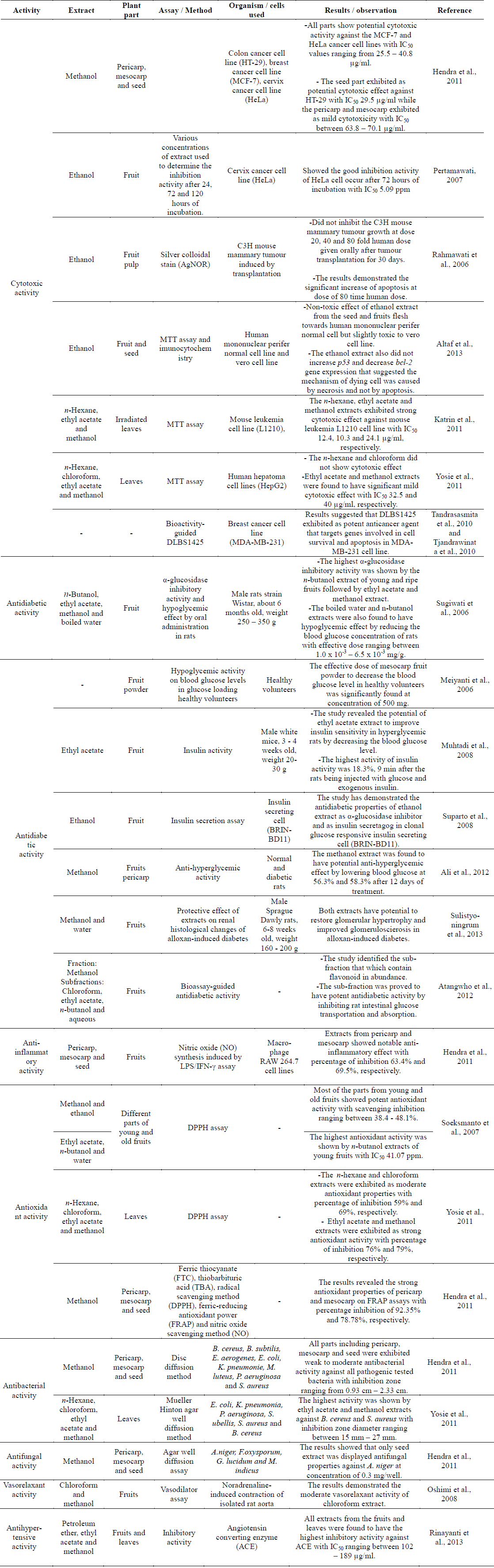
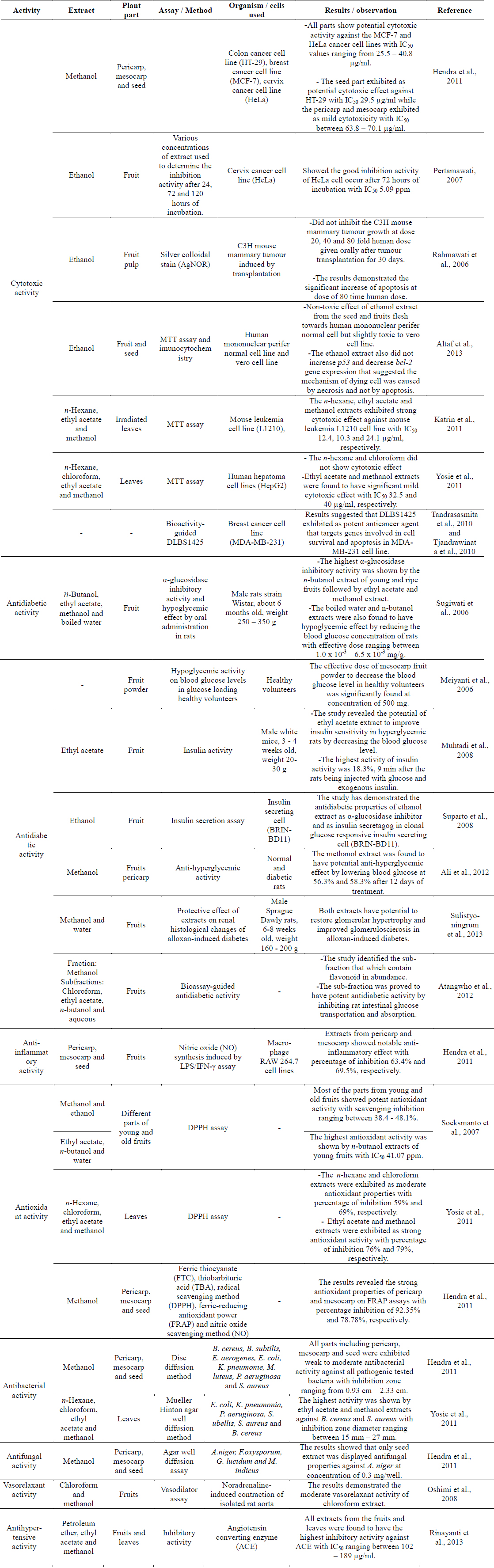
Cytotoxic activities of the methanolic extract from different parts of P. macrocarpa fruits were evaluated against the human colon adenocarcinoma cell line (HT-29), human breast adenocarcinoma cell line (MCF-7), human cervical cell line (HeLa) and normal human hepatocytes cell line (Chang liver cell). The viability of cells was measured using the MTT assay. The fruits were divided into pericarp, mesocarp and seed. Results obtained indicated that all parts had potential cytotoxic activity against the MCF-7 and HeLa cancer cell lines with IC50 values ranging from 25.5 – 40.8 μg/ml. The results also showed that the seeds exhibited potential cytotoxic effect against HT-29 with an IC50 value of 29.5 μg/ml while the pericarp and mesocarp exhibited mild cytotoxicity with IC50 values between 63.8 – 70.1 μg/ml (Hendra et al., 2011).
An in vitro study on the cytotoxic effect of fruit extract was carried out against the human uterine cervical carcinoma cell line (HeLa) (Rahmawati et al., 2006). Various concentrations of fruit extract were used to determine the inhibition activity against the HeLa cell line after 24, 72 and 120 h of incubation. The results showed good inhibitory activity against the HeLa cell after 72 h of incubation with an IC50 value of 5.09 ppm (Pertamawati, 2007). Another investigation on the anticancer activity of the ethanol extract of the fruit pulps of P. macrocarpa against mouse mammary tumour was induced by transplantation. This study concluded that the ethanol extract did not inhibit the mouse mammary tumour growth at doses of 20, 40 and 80 fold human doses given orally after tumour transplantation for 30 days. However, the results demonstrated the significant increase of apoptosis atthe dose of 80 time human dose (Rahmawati et al., 2006).
Previous cytotoxic study on the fruits of P. macrocarpa reported the non-toxic effect of the ethanol extract from the seeds and fruits flesh towards human mononuclear peripheral normal cell but slightly toxic to the vero cell line. The extract also did not increase p53 and decrease bcl-2 gene expression that suggested the mechanism of dying cell was caused by necrosis and not by apoptosis (Altaf R et al., 2013). Bioactivity study of irradiated P. macrocarpa leaves showed that the n-hexane, ethyl acetate and methanol extracts exhibited strong cytotoxic effect against the mouse leukaemia L1210 cell line with IC50 values of 12.4 10.3 and 24.1 μg/ml, respectively (Katrin et al., 2011). In addition, cytotoxic activity of the n-hexane, chloroform, ethyl acetate and methanol extracts from the leaves of P. macrocarpa plant were investigated against the human hepatoma cell lines (HepG2). The ethyl acetate and methanol extracts were found to have mild cytotoxic effect (IC50 32.5 and 40 μg/ml, respectively) (Yosie et al., 2011).
The investigations on cytotoxic effects of isolated compounds were conducted against several cancer cell lines. Study on cytotoxic activity of phalerin (1) from the methanolic extract of P. macrocarpa leaves was investigated against the myeloma cell line (NS-1). Phalerin (1) was non-toxic towards NS-1 cell line (IC50 83μg/ml) (Altaf et al., 2013). Two benzophenone glucosides, mahkoside A (4) and mahkoside B (5) were found to have low cytotoxic effect towards several human cancer cell lines including the prostate cancer cell line (PC-3), stomach cancer cell line (MGC-803) and esophageal cancer cell lines (EC109 and EC9706), with IC50 values exceeding 100 μM/L (Zhang et al., 2012). However, the inhibitory activity of another benzophenone glucoside named as 6,4'-dihydroxy-4-methoxybenzophenone-2-O-β-D-gluco pyranoside (7) displayed that this compound had strong cytotoxic properties against the mouse leukaemia cell line (L1210) (IC50 5.1 μg/ml) (Winarno and Katrin, 2009). Additionally, fevicordin A (15) demonstrated strong anticancer properties against the murine leukaemia cell line (P388) and cervix cancer cell line (HeLa) (IC50 0.01 and 1.16 μg/ml, respectively). This compound also exhibited moderate anticancer activity against another cervix cancer cell line (CasKi) and oesophagus cancer cell line (TE-8) with IC50 values of 12 and 14.6 μg/ml, respectively (Diantini et al., 2012).
Faried et al. (2007) studied the anticancer properties of gallic acid (25), isolated from the fruits of P. macrocarpa. The cell proliferation activity was performed using the MTT assay against the human esophageal cancer cell line (TE-2), gastric cancer cell line (MKN-28), colon cancer cell lines (HT-29 and Colo201), breast cancer cell line (MCF-7), cervix cancer cellline (CaSki), mouse colon cancer cell line (colon 26) with one normal human esophageal cell line (CHEK-1). Interestingly, gallic acid (25) showed significant anticancer properties towards all tested cancer cell lines and induced apoptosis in TE-2 cell line. Apart from the cytotoxic screening activities, the study on molecular mechanism of the extract from this plant on human breast cancer cell line (MDA-MB-231) was performed using a bioactivity-guided DLBS1425. In a previous study, DLBS1425 was found to confer antiproliferative and proapoptosis effects via eicosanoid pathway (Tjandrawinata et al., 2010). DLBS1425 was shown as a potent anticancer agent that targets genes involved in cell survival and apoptosis in the MDA-MB-231 cell line (Tandrasasmita et al., 2010).
The investigation on the α-glucosidase inhibitory activity and hypoglycemic effect by oral administration of fruit extracts from P. macrocarpa in rats were evaluated (Sugiwati et al., 2006). The highest α-glucosidase inhibitory activity was displayed by the n-butanol extract of young and ripe fruits followed by the ethyl acetate and methanol extracts. The boiled water and n-butanol extracts also displayed hypoglycemic effect by reducing the blood glucose concentration of rats with effective dose ranging between 1.0 × 10-3 – 6.5 × 10-3 mg/g. The results suggested that this plant could be suitable as a traditional antidiabetic drug (Sugiwati et al., 2006). The hypoglycemic activity of this plant was further investigated by evaluating the effect of the fruits powder on blood glucose levels in glucose loading healthy volunteers. The effective dose of mesocarp fruit powder to decrease the blood glucose level in healthy volunteers was significantly found at the concentration of 500 mg (Meiyanti et al., 2006).
Another study revealed the potential of the ethyl acetate extract of fruits to improve insulin sensitivity in hyperglycemic rats by decreasing the blood glucose level. The highest activity of insulin activity was 18.3%, 9 min after the rats being injected with glucose and exogenous insulin (Muhtadi et al., 2008). The in vitro mechanism study demonstrated the antidiabetic properties of the ethanol extract of the fruits as α-glucosidase inhibitor and as insulin secretagog in clonal glucose responsive insulin secreting cell (BRIN-BD11). In the same study, the qualitative analysis of phytochemicals from the ethanol extract revealed the presence of flavonoid, alkaloid, tannin and steroid. Among these classes of compounds, flavonoid was suggested to be responsible for the antidiabetic properties of the extract in that study (Suparto et al., 2008). In addition, the methanol extract from the fruits pericarp was found to have potential anti-hyperglycemic effect by lowering blood glucose at 56.3% and 58.3% after 12 days of treatment.
This finding led to phytochemical screening, revealing the presence of flavonoids, terpenoids, and tannins in the methanol extract. These classes of compounds were suggested to be the major contributors to its antidiabetic properties (Ali et al., 2012).
Sulistyoningrum et al. (2013) discovered the protective effect of the methanol and water extracts of P. macrocarpa on renal histological changes of alloxan-induced diabetes. The results concluded that both extracts were able to restore glomerular hypertrophy and improved glomeruloscierosis in alloxan-induced diabetes. Bioassay-guided antidiabetic study on the extract from the fruits successfully identified the active sub-fraction that had flavonoids in abundance. The sub-fraction was proved to have potent antidiabetic activity by inhibiting rat intestinal glucose transportation and absorption. (Atangwho et al., 2012).
Different parts of the fruits of P. macrocarpa were screened for their anti-inflammatory activity using the nitric oxide (NO) synthesis in macrophage RAW 264.7 cell lines induced by the LPS/IFN-γ assay. Extracts from the pericarp and mesocarp showed notable anti-inflammatory effect with percentage of inhibition of 63.4% and 69.5%, respectively (Hendra et al., 2011). Study on anti-inflammatory activity was performed on the major compound from the fruits identified as phalerin (1). This compound showed low inflammatory effect since it decreased the inflammation twice lower than the standard, Napoxen at dose of 22.5 mg/kg body weight (Mariani et al., 2010). Anti-inflammatory activity of phalerin (1) was also determined by using the lipoxygenase (LOX), hyaluronidase (HYA) and xanthine oxidase (XO) assays. The results showed that phalerin (1) had mild anti-inflammatory properties in the XO and LOX assays with percentage of inhibition 34.8% and 23.5%, respectively. Meanwhile, phalerin (1) did not exhibit any inflammatory effect in the HYA assay (Fariza et al., 2012).
Antioxidant activity of the methanol and ethanol extracts from different parts of young and old fruits of P. macrocarpa was evaluated using the free-radical-scavenging method (DPPH). Most of the parts from young and old fruits showed potent antioxidant activity with scavenging inhibition ranging between 38.4 - 48.1%. Further fractionation of the active extracts was carried out to give ethyl acetate, n-butanol and water extracts. The highest antioxidant activity was observed in the n-butanol extract of the young fruits with IC50 of 41.07 ppm (Soeksmanto et al., 2007). The same DPPH method was also performed to determine the antioxidant activity of different polarity of extracts from the leaves of P. macrocarpa. Ethyl acetate and methanol extracts exhibited strong antioxidant activity with 76% and 79% inhibitions, respectively. Meanwhile, the n-hexane and chloroform extracts displayed moderate antioxidant properties with 59% and 69% inhibitions, respectively (Yosie et al., 2011).
Various in vitro model systems such as ferric thiocyanate, thiobarbituric acid, DPPH, ferric-reducing antioxidant power (FRAP) and nitric oxide (NO) scavenging method were used to characterize the antioxidant properties of different parts of the fruits. The results revealed the strong antioxidant properties of the pericarp and mesocarp in the FRAP assays with percentage inhibition of 92.35% and 78.78%, respectively (Hendra et al., 2011). In addition, antioxidant study of 2,6,4'-trihydroxy-4-methoxybenzophenone (8) and macronone (26) performed using the DPPH method. Interestingly, compound (8) displayed strong antioxidant properties (IC50 10.57 μg/ml) while macronone (26) had weak activity (IC50 240.14 μg/ml) (Susilawati et al., 2011; 2012).
Antibacterial activity of various parts of P. macrocarpa fruits was studied using the disc diffusion method against eight bacterial strains, i.e., Bacillus cereus, Bacillus subtilis, Enterobacter aerogenes, Escherichia coli, Klebsiela pneumonie, Micrococcus luteus, Pseodomonas aeruginosa and Staphylococcus aureus. All parts including the pericarp, mesocarp and seeds exhibited weak to moderate antibacterial activity against all pathogenic bacteria strains with inhibition zones ranging from 9.3 – 23.3 mm (Hendra et al., 2011). In the same study, the antifungal activity was evaluated using the agar well diffusion assay against Aspergillus niger, Fusarium oxysporum, Ganoderma lucidum and Mucor indicus. The results showed that only seed extract was active against A. niger at a concentration of 0.3 mg/well (Hendra et al., 2011).
Different polarities of extracts from the leaves of P. macrocarpa including the n-hexane, chloroform, ethyl acetate and methanol extracts were evaluated for their antibacterial activity against E. coli, K. pneumonia, P. aeruginosa, Streptococcus ubellis, Streptococcus aureus and B. cereus. Mueller Hinton agar well diffusion method was used to determine the susceptibility of bacteria tests. The highest activity was shown by ethyl acetate and methanol extracts against B. cereus and S. aureus with inhibition zone diameter ranging between 1527 mm (Yosie et al., 2011).
The general toxicity of 29-norcucurbitacin derivatives; desacetyl-fevicordin A (14), fevicordin A (15), fevicordin A glucoside (16) and fevicordin D glucoside (17), isolated from this plant was evaluated by the brine shrimp (Artemia salina) lethality assay. All compounds showed variable general toxicity with LD50 values ranging from 3 – 12 ppm (Kurnia et al., 2008).
The vasorelaxant activity of the extracts and compounds (1, 9, 28) isolated from the fruits of P. macrocarpa was evaluated against noradrenaline-induced contraction of isolated rat aorta. The results demonstrated the moderate vasorelaxant activity of the chloroform extract, while icariside C3 (9) showed a slow vasorelaxant activity. Phalerin (1) and mangiferin (28) did not show any vasorelaxant effect (Oshimi et al., 2008).
Study on the antihypertensive activity of nine medicinal plants from Indonesia was conducted against angiotensin converting enzyme. The nine tested plants were Scurulla artopurpurea, catharanthus roseus, Swietenia mahogany, Persea Americana, Oxalis corniculata, P. macrocarpa, Gynura procumbens, Melia azedarach and Hisbiscus rosasinensisi. Interestingly, all extracts from the fruits and leaves of P. macrocarpa displayed the highest level of inhibitory activity against acetylcholine esterase with IC50 ranging between 102 – 189 μg/ml (Rinayanti et al., 2013).
In this review, we have reviewed the relevant literature to assemble the ethnomedicinal, phytochemical and pharmacological properties of P. macrocarpa (Scheff). Boerl. This plant is used as folk remedies in both traditional as well as modern system of medicine to treat various diseases and illnesses. Various types of compounds with diverse chemical structures present in this plant are responsible for varied pharmacological and medicinal properties. Reported data show that the plant possesses promising anticancer, antidiabetic, antiinflammatory, antioxidant, antimicrobial, antihypertensive, toxicity and vasorelaxant activities. However, in view of the wide range of medicinal uses of P. macrocarpa, it is necessary to conduct further clinical and pharmacological studies at molecular level to investigate the potential of this plant, because most of the activity reported is based only on their in vitro assays. Similarly, additional studies have to be carried out in order to establish the potential of the extracts of P. macrocarpa in the development of new therapeutic drugs and to provide the basis for future research on the application of medicinal plants.