Fumaria indica Linn. (Syn: Fumaria parviflora, Fumariaceae) is a wildly grown weed, mentioned and recommended in classical Ayurvedic texts for treatments of variety of ailments including dermatological diseases, topical diseases, cardiovascular complaints, circulatory disease, fever and headache etc. The present pilot study was designed to experimentally verify the possibility that fumarates are the major bioactive principles of Fumaria indica extracts involved in their stress response modulating activities, and to estimate pharmacologically active dose ranges of fumarates and standardized methanolic extract of Fumaria indica (MFI). Effect of single, 5 and 10 daily oral doses of pure fumaric acid (FA), monomethyl fumarate (MMF), dimethyl fumarate (DMF) and MFI was quantified in well validated rodent models viz. apomorphine induced cage climbing, stress induced hyperthermia, and elevated plus-maze tests. Obtained results reveal high efficacy of MFI and pure fumarates possess qualitatively analogous activity profiles in all the three tests. There were no significant difference in the potencies of pure FA, MMF and DMF in the three tests, whereas efficacy of MFI in the elevated plus maze test for anxiolytics was higher than in the other two tests. Efficacies of all the four test agents in all the three tests increased with increasing number of days of oral treatments. Results of these pilot experiments should be helpful for more rational selections of pharmacologically interesting dose ranges and treatment regimens of fumarates and Fumaria indica extracts for further more holistic explorations of their diverse therapeutic potentials.
Fumaric acid is a structurally simple metabolic intermediate of all living cells now well recognized as a functionally important fixed carbon source of many terrestrial plants (Araújo et al., 2011; Chia et al., 2000). It was first isolated from a fumitory plant (
Such is also the case for the Ayurvedic medicinal plant
However, the question whether fumaric acid or its hydrolysable conjugates are the major bioactive constituents of medicinally used
The standardized 50% methanolic extract of
Fumaric acid (FA), mono-methyl fumarate (MMF) and di-methyl fumarate (DMF) were procured from Sigma-Aldrich, USA. All other chemicals and reagents used were obtained from local commercial sources and were of best commercial quality available in India.
Adult Charles Foster albino rats (150 ± 10 g) and Wistar mice (20 ± 5 g), of either sex, were housed in groups of six in polypropylene cages at an ambient temperature of 25℃ ± 1℃ and 45-55% relative humidity, with a 12 : 12 h light/dark cycle. Unless stated otherwise, the animals were always provided with commercial food pellets and water
All test substances were suspended in 0.3% w/v carboxy methyl cellulose (CMC) for oral administrations. Application volumes in all cases were 10 ml/kg, and the control animals were always treated accordingly with 10 ml/kg of 0.3% w/v CMC. The very first daily doses of test agents, or of the vehicle, were administered one hour before the start of other experimental procedures, and the same experimental procedures were repeated after once daily oral treatments for five and ten consecutive days. The daily oral doses of FA, MMF, and DMF used in all tests were 2, 6, 18, and 54 mg/kg, whereas that of MFI were 60, 120, 240 and 480 mg/kg/day. For different tests, different groups of naive animals were used.
>
Stress induced hyperthermia (SIH) test
Transient hyperthermia is a global response to a stressful condition. Stress-induced hyperthermia test is often used as a model for quantifying anxiety state of rodents (Groenink et al., 2009). In this study, foot shock treatment was used as paradigm of stress, which causes transient as well as long term increases in core body temperature via involvement of hypothalamic process (Moreno, 2010). In this test, mice were placed in a black box (24 × 29 × 40 cm) with a grid floor for 1 min. Foot shock stress was delivered by electric shock through the grid floor (2 mA, 50 H of 2 ms duration), and five consecutive foot shocks of 2 mA at 10 s intervals were delivered after their 10 s stay in the cage. At the end of one minute stay of the animals in such cages, they were manually placed back in their home cages. Stress induced hyperthermia (∆T) in mice was quantified by subtracting their rectal temperatures measured just before subjecting them to footshock stress (BT), from their rectal temperatures measured 10 min after the footshock stress (FT) by using calibrated rectal thermometer (Van der Heyden et al., 1997; Vinkers et al., 2008; Zethof et al., 1994).
>
Apomorphine induced cage climbing test
Apomorphine is a potent and specific agonist of dopaminergic receptor, and causes stereotype hyperactivity. Stereotyped cage-climbing behaviour induced by apomorphine was quantified according to the procedure reported by Protais et al. (1976) with some modifications (Davis et al., 1986). Apomorphine (0.50 mg/kg, dissolved in 0.1% sodium meta-bisulphite solution, s.c.) was administered after 60 min of test agents treatment, and immediately after this challenge a rat was individually put into a cylindrical cage of 20 cm in diameter and 40 cm high. The walls of the cages were made of vertical metal bars (2 mm in diameter) fixed 1 cm apart with a smooth upper metal ring. After a 5-min-period of exploratory behaviour, two consecutive observations were performed on each animal by a blind observer at 10 and 20 min after apomorphine injection and these two scores were averaged. Behaviours of the animals were scored as follows: 0 = behaviour indistinguishable from that of normal (vehicle alone) rats, 1 = increased locomotor activity, pacing, tail stiffening, some sniffing, 2 = addition of occasional intermittent clinging to the sides of the cage with forepaws, 3 = addition of intermittent clinging with hind paws as well as forepaws, 4 = intense, virtually uninterrupted clinging to the sides or top of the cage with all paws (Baldessarini et al., 1977).
>
Elevated plus-maze (EPM) test
The EPM model is the most commonly used rodent behavioural model for anxiety. Anxiety reduction in the plus-maze is indicated by an increase in time spent and number of entries in open arms and decrease in time spent and number of entries in enclosed arms. The method of Pellow and File (1986) was followed. The maze used in this study had two opposite arms, 50x10 cm, crossed with two enclosed arms of the same dimension but having 40 cm high walls. The arms were connected with a central square, 10x10 cm, giving the apparatus a shape of a plus sign. The maze was kept in a dimly lit room and elevated 50 cm above the floor. Naive rats were placed individually in the centre of the maze, facing an enclosed arm. Thereafter, number of entries and time spent on the open and enclosed arms was recorded during the next 5 min. An arm entry was defined when all four paws of the rat were in the arm. A neutral ‘blind’ observer made observations (Kumar et al., 2000).
Mean ± standard error of mean (SEM) were calculated for the observed values in each experimental group. Statistical analysis was performed by two way analysis of variance (ANOVA) followed by Bonferroni post tests, unless stated otherwise. GraphPad Prism 5 (GraphPad Software Inc., CA, USA) was used for statistical analysis and calculating ED50 values.
>
Stress induced hyperthermia (SIH) test
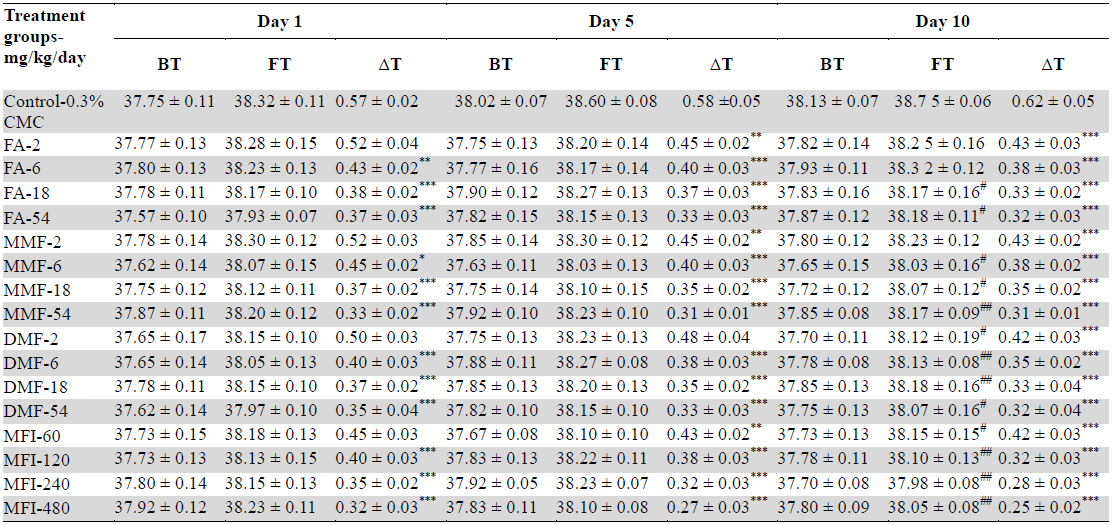
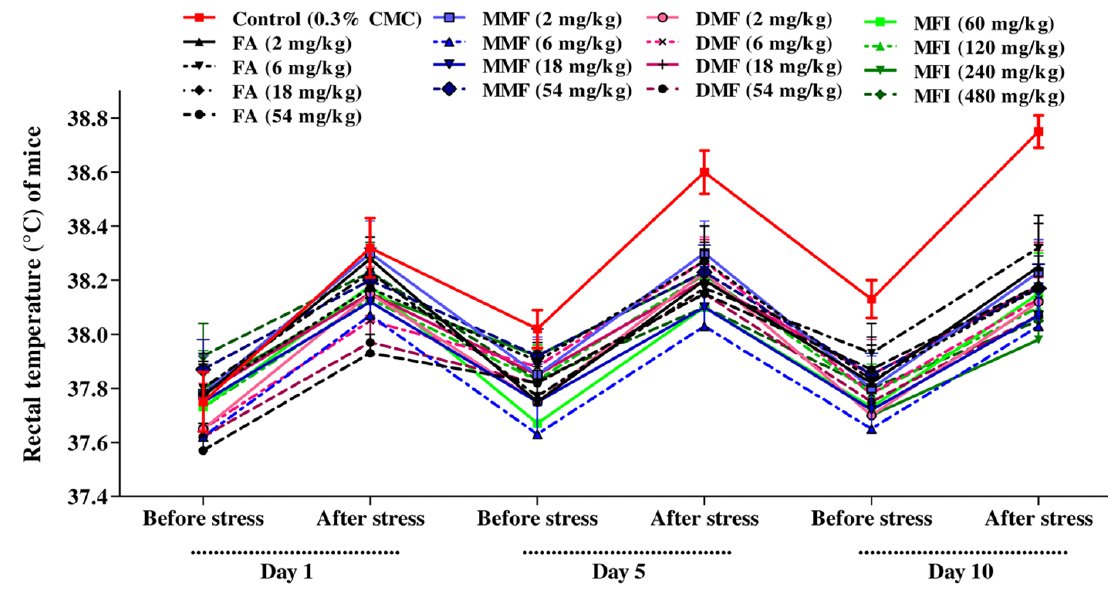
Mean rectal temperatures of different groups recorded immediately before foot shock exposures (BT) and after that (FT) recorded on days 1, 5 and 10 of the test are summarised in Table 1 and Fig. 1. It is apparent from these values that on the first day of the test no statistically significant effects of treatments on BT with any of the test doses of FA, MMF, DMF or MFI on basal core temperature were observed. Such were not the cases on the 5th and 10th days of the test. It was also interesting to note that BTs of the vehicle treated control group on these two days were somewhat higher than that recorded for the group on the first test day, whereas those of all test agents treated groups remained almost constant. These observations reveal that even the lowest tested doses (2 mg/kg/day) of FA, MMF, DMF, or of 60 mg/kg/day MMF completely suppress the long term elevations of basal rectal temperatures of mice subjected to one or two sessions of foot shock stress of 1 min duration on the first and fifth days of the experiments.
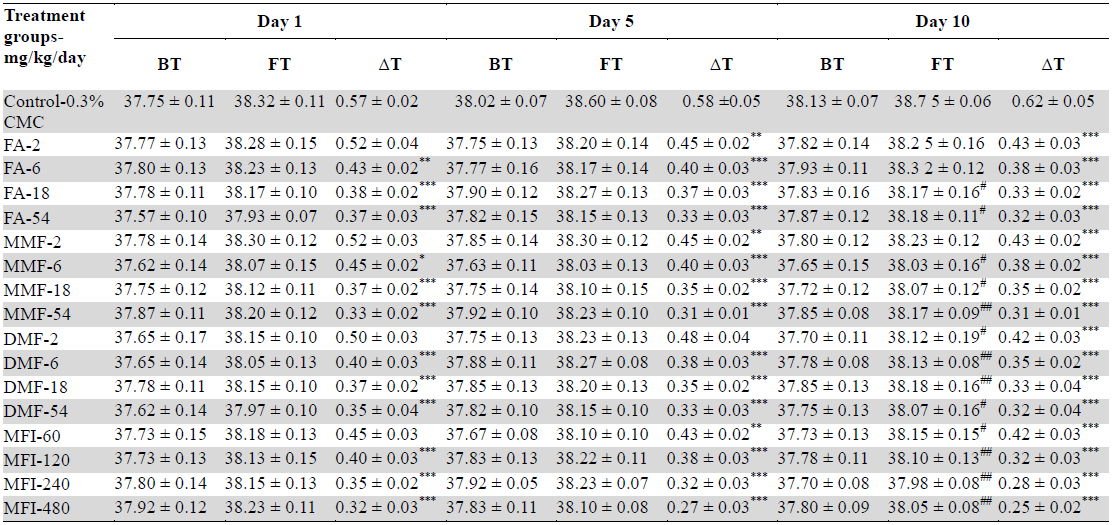
Effects of single, five and ten daily oral doses of fumaric acid (FA), monomethyl fumarate (MMF), dimethyl fumarate (DMF), and methanolic Fumaria indica extract (MFI) on basal rectal temperatures (BT) and on rectal temperatures recorded 10 minutes after stress exposures (FT) in mice foot shock stress induced hyperthermia test.
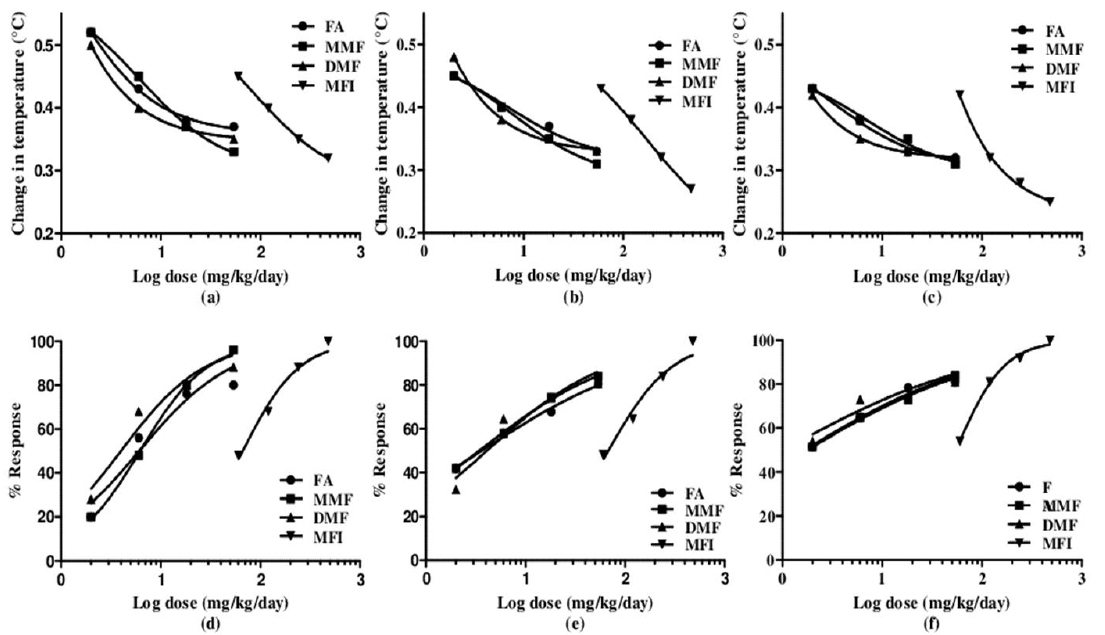
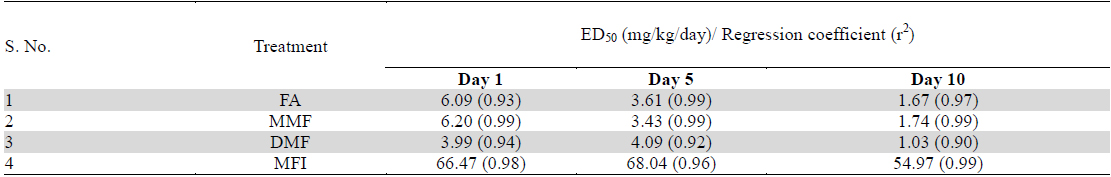
Despite higher BT values of the vehicle treated group on days 5 and 10 of the test, the magnitude of foot shock stress induced transient hyperthermia observed in this group remained almost constant on all the three test days. From the dose response curves shown in Fig. 2 it is apparent that all the four tested agents dose dependently inhibited the transient hyperthermic responses triggered by one minute duration of foot shocks on all the three observational days, Hereupon the efficacies of FA, MMF, and DMF on all the three observational days were almost identical, and their dose dependent efficacies increased somewhat with increasing numbers to treatment days. Statistically significant minimal effective doses of FA, MMF and DMF after their 10 daily oral doses were lower than 2 mg/kg/day, whereas that after their single oral doses on day 1 was about 4 mg/kg. Analogous were the observed effects of MFI. On day 1, statistically significant minimal effective dose of MFI was 120 mg/kg, whereas on days 5 and 10 this was 60 mg/kg. It is apparent from the Fig. 2a-c that dose dependent efficacies of all test agents in suppressing stress triggered transient hyperthermia on the first day of the experiments were always lower than those observed on the 5th and 10th days. ED50 (effective dose which gives 50% effect to corresponding maximum response) values of single, 5 and 10 daily oral dose of FA, MM, DMF and MFI calculated by nonlinear regression using log dose vs. normalized response-variable slop Fig. 2 d-f by GraphPad Prism 5, are given in Table 2.
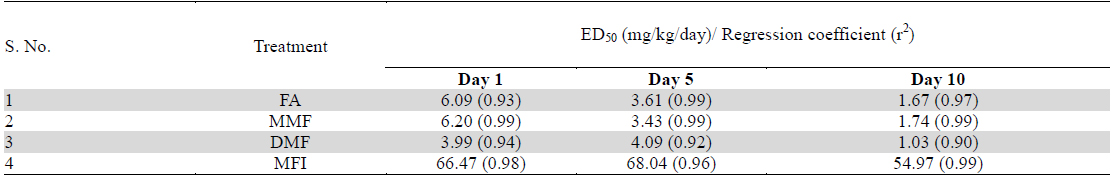
Calculated ED50 values of pure fumaric acid (FA), monomethyl fumarate (MMF), dimethyl fumarate (DMF) and methanolic extract of Fumaria indica (MFI) for inhibiting foot shock stress triggered transient hyperthermia in mice. These values were calculated by nonlinear regression analysis using experimental data summarised in Table 1.
>
Apomorphine induced cage climbing test
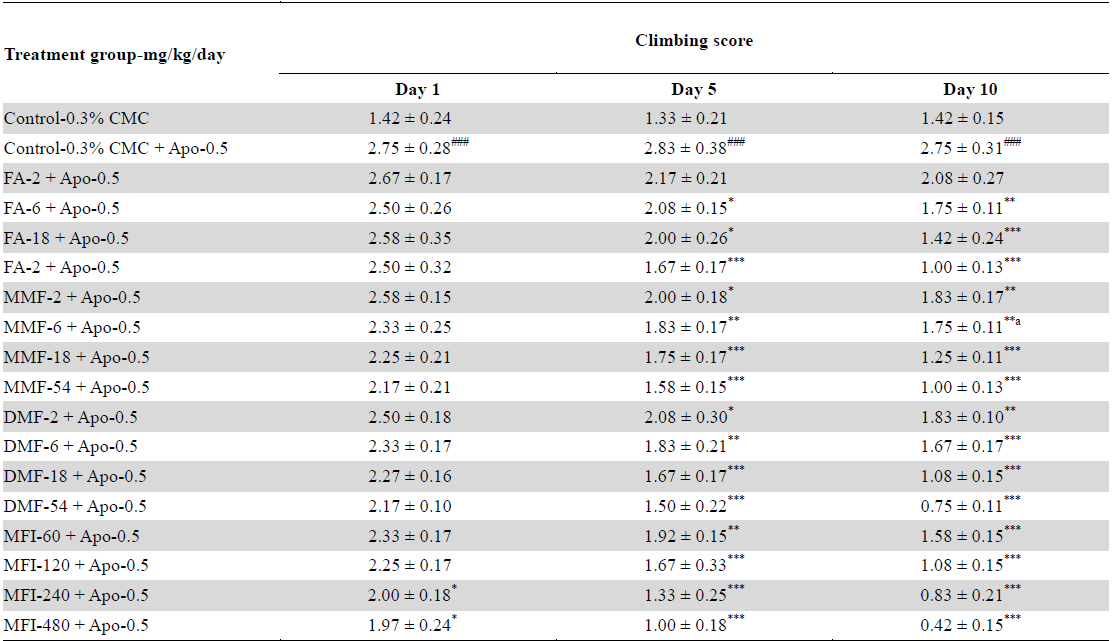
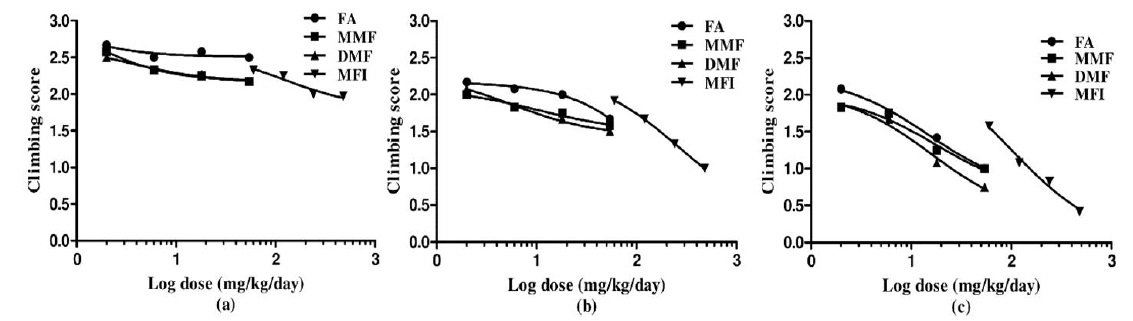
Outcome of cage-climbing behaviour summarized in Table 3 reveal that apomorphine treated control animals had significantly higher scores than CMC treated control ones, and that no significant effects of the test agents were observed after their single oral doses. After 5 daily oral treatments significant inhibitory effects only of the highest dose of DMF (54 mg/kg/day, and of the two highest ones of the MFI (240 and 480 mg/kg/day) were observed. However, after 10 daily treatments, clear dose dependant inhibitory effects of all test agents were observed. Their dose response curves derived from the data summarized in Table 3 are shown in Fig. 3. It is apparent from these curves that the effects of FA, MMF and DMF treatments are almost identical on each of the three observational days, and that their statistically significant and dose dependant effects were detectable after their repeated daily doses only. Hereupon their efficacies on observed on the 10th observational day were much higher than those recorded on the 5th one. Quite analogous were also the observed effects of MFI in its tested dose range.
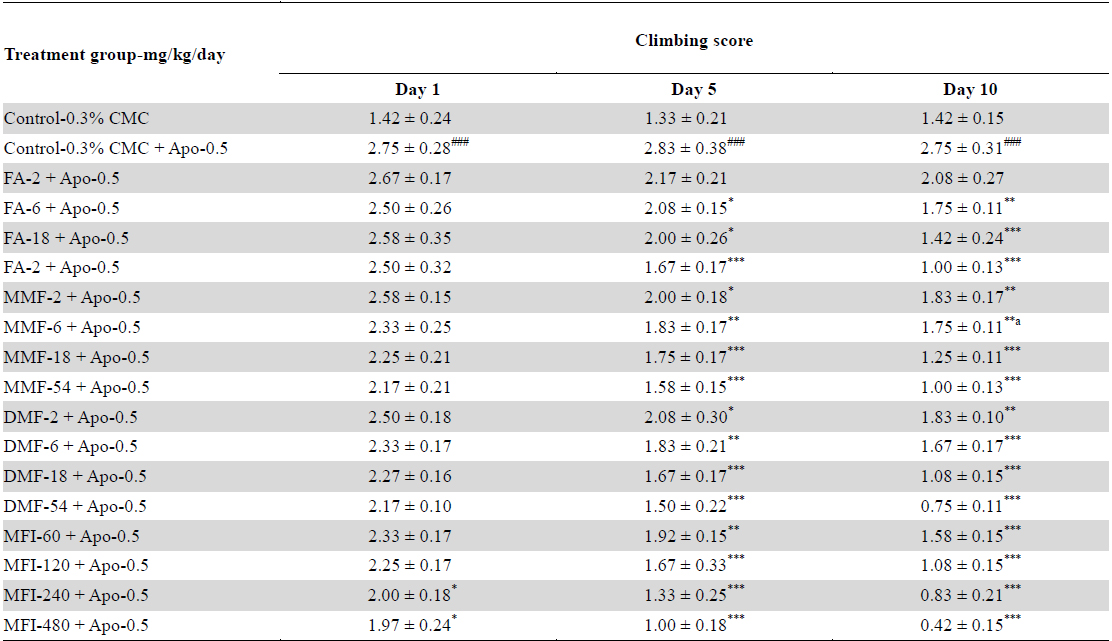
Effects of single, five and ten daily oral doses of fumaric acid (FA), monomethyl fumarate (MMF), dimethyl fumarate (DMF), and methanolic extract of Fumaria indica (MFI) on apomorphine (0.5 mg/kg) induced climbing behaviour of rats in cage climbing test.
>
Elevated plus-maze (EPM) test
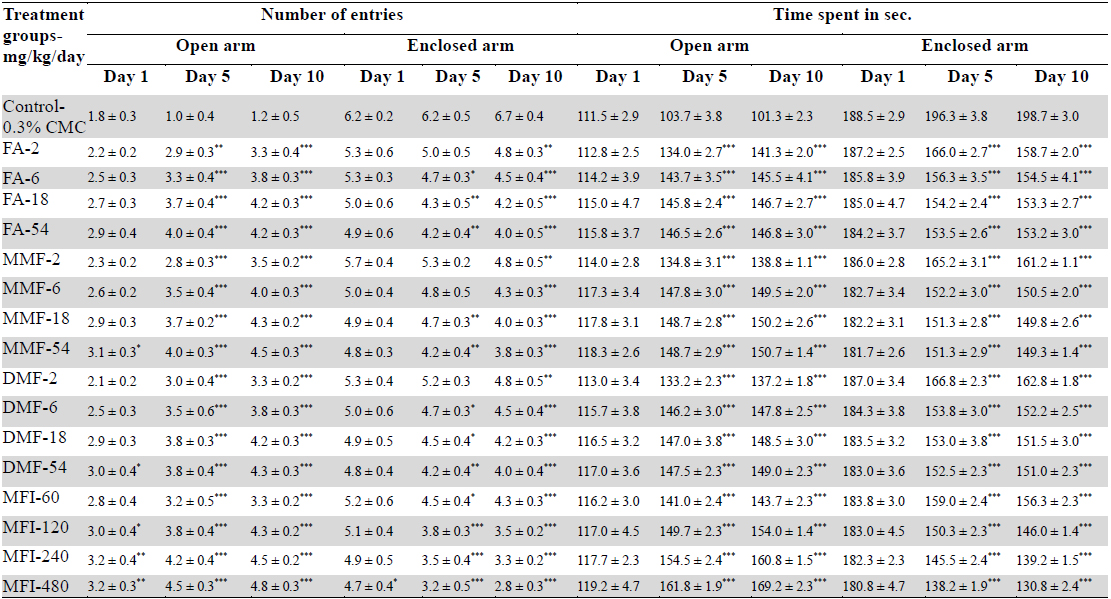
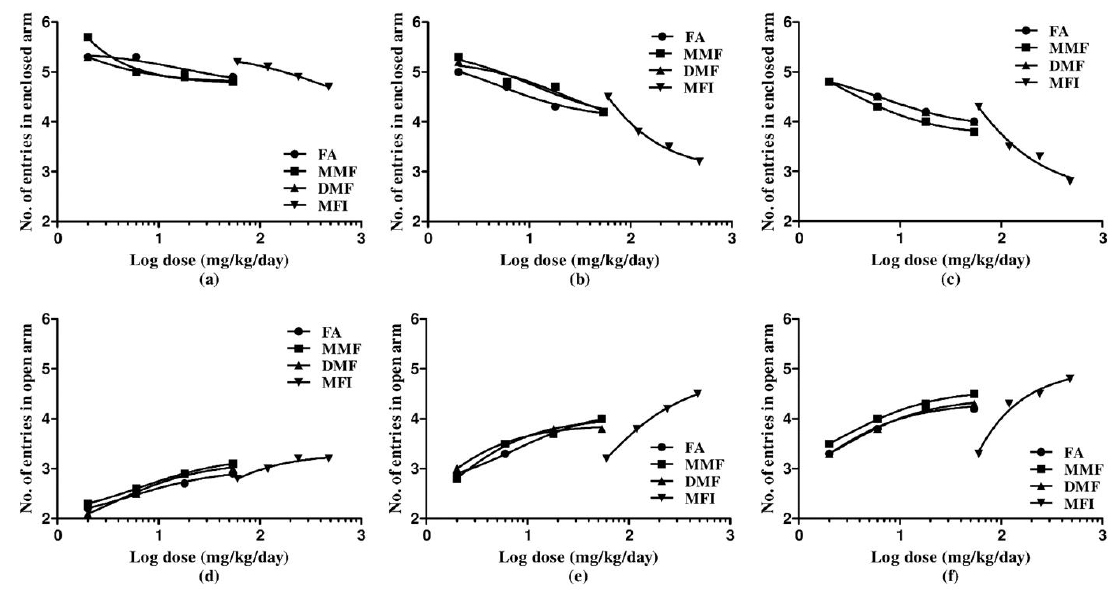
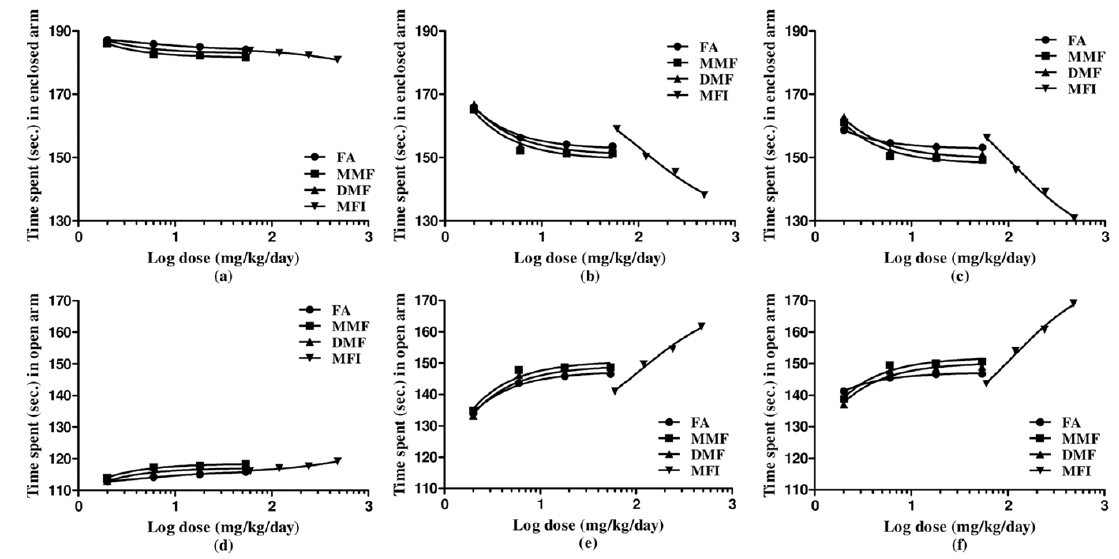
Results of the experiments comparing the effects of FA, MMF, DMF and MFI in this test are summarised in Table 4, and for clarity sake dose response curves of their observed effects on the four parameters quantified are shown in Fig. 4-5. Dose and duration of treatment dependant anxiolytic efficacy of all the four test agents were observed. No statistically significant effects of FA on any of the quantified parameters were observed after its single oral doses, whereas single highest doses of MMF (54 mg/kg), DMF (54 mg/kg) and MFI (480 mg/kg) tested significantly increased the number of entries in the open arm of the maze. However, after their 5 and 10 daily oral doses statistically significant and dose dependant anxiolytics like efficacies of all the four test agents were observed. Like in other two other behavioural tests used in this pilot study, their efficacies in this test observed on the 5th treatment days were also always lower than those observed after their 10 daily doses. Similarly, like in other two tests used, the dose response curves of FA, MMF, and DMF on all the three observational days were almost identical; they seem to be equi-effective brain function modulating agents. On all the three test days, the anxiolytic like efficacy of MFI observed after its tested dose range was always higher than those observed of the tested dose ranges of pure FA, MMF, and DMF.
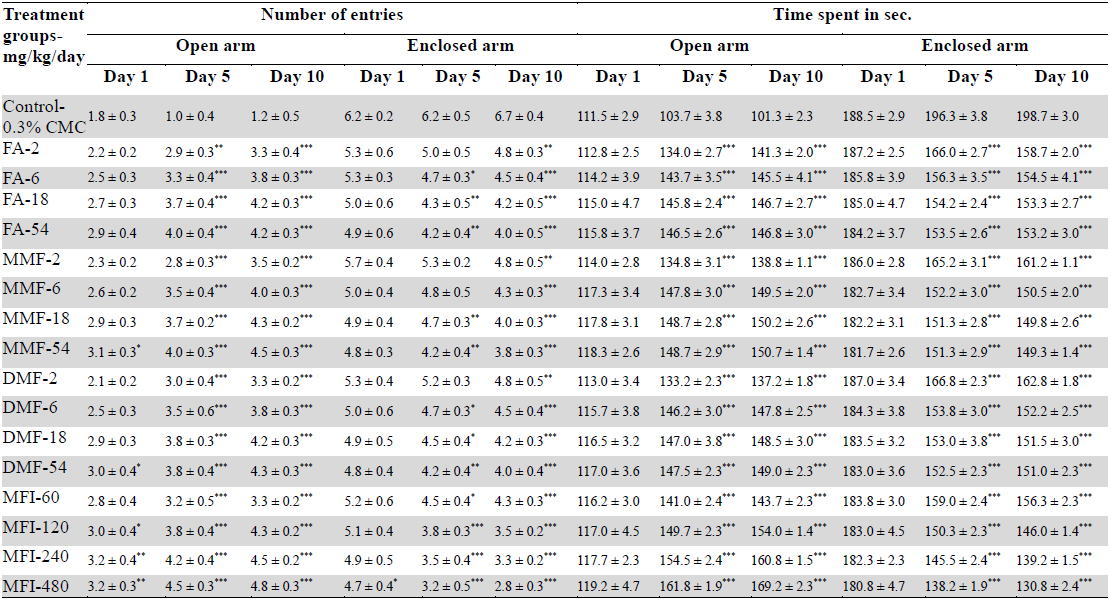
Effect of single, five and ten daily oral doses of fumaric acid (FA), monomethyl fumarate (MMF), dimethyl fumarate (DMF), and methanolic extract of Fumaria indica (MFI) on number of entries and time spent in open and enclosed arms in rat elevated plus maze test.
Observations reported in this communication not only add further experimental evidences in favour of the conviction that fumaric acid and its hydrolysable conjugates are the quantitatively the major adaptogenic constituents of hydro alcoholic
Although analogous activity profiles of MFI was observed in all the three tests, its daily oral dose dependant efficacy in the EPM test for anxiolytics cannot be explained by its analytically estimated contents of fumaric acid and its conjugates. The calculated daily oral doses of total fumarates (i.e. sum of the contents of free acid and its acid hydrolysable conjugates) administered with daily 60, 120, 240, and 480 mg/kg doses of the extract were 0.64, 1.28, 2.57, and 5.14 mg/kg respectively. Although dose dependant effects of all the three fumarates tested were observed, their efficacies observed in the test after their highest doses tested (54 mg/kg/day) were quantitatively almost equal to those observed after the lowest MFI doses (60 mg/kg/day), which delivered only 0.64 mg/kg daily doses of total fumarates. Since such differences were not observed in the apomorphine test, it could seems reasonable to assume that anti-dopaminergic and anxiolytic effects of the test agents are two independent effects and that MFI must have some other bioactive constituents with anxiolytic as well as anti-dopaminergic activities. However, further efforts will be necessary for more definitive inferences from the observation made during this pilot study.
Dysfunctions of central dopaminergic neurotransmission together with those of diverse others neurotransmitter systems are hall marks of diverse spectrums of mental health problems commonly associated with lifestyle and environmental stress triggered pathologies (Alghasham and Rasheed, 2014; Christmas et al., 2008; Furmark, 2009; Nikolaus et al., 2010). Since currently known psychoactive and other drugs have adverse effects and do not often meet the therapeutic demands of mentally ill patients, efforts are now being made in many laboratories to identify novel therapeutic leads from psychoactive and other plants. Although number of reports suggesting anxiolytic potentials of numerous plants have consistently increased during more recent years, as yet little concentrated efforts have been made to obtain analytically as well pharmacologically well standardised extracts necessary for further developments according to current concepts of evidence based medicine (Gelfuso et al., 2014; Lakhan and Vieira, 2010; Sharma et al., 2012). Prior knowledge of the bioactive constituents of plants, and their pharmacologically relevant
dose ranges and treatment regimen is an essential prerequisite for such ventures. Taken together with our earlier observations (Singh et al., 2012a, 2012b, 2013a), the ones reported in this communication reveal only that total contents of fumaric acid and its hydrolysable conjugates are bioactive secondary metabolite of the plant, and strongly suggest that brain function modulating effects of MMF and DMF are most probably due to their rapid hydrolysis (inside the gastrointestinal tract or elsewhere) to fumaric acid. These later mentioned inference, and a recent report revealing that DMF has protective effects against central dopaminergic toxicity (Jing et al., 2015), strongly suggest that some of the clinically observed beneficial effects of the di-ester in patients suffering from psoriasis or multiple sclerosis, could as well be due to its central dopaminergic effects, and that fumaric acid itself could be a better tolerated therapeutic option for health problems caused by central dopaminergic abnormalities.
Although several more recent reports continue to point out diverse therapeutic potentials of fumarates (Ellrichmann et al., 2011; Ermis et al., 2013; Gkalpakiotis et al., 2014; Seidel and Roth, 2013; ilhavý et al., 2014; Strassburger-Krogias et al., 2014), most of them deal only with DMF, and as yet no reports on therapeutic potentials of repeated low oral doses of fumarates have appeared. Our observations revealing that even 2 mg/kg daily oral doses of fumaric acid and its esters afford protection again against transient as well as long term effects of stress triggered central thermoregulatory and dopaminergic processes, strongly suggest that low dose fumarates could be a therapeutic or preventive alternative against central sensitivity syndromes commonly associated with almost all chronic inflammatory disorders. They not only add further experimental evidences in support of our earlier analogous speculative suggestions (Shakya et al., 2014), and suggest that modulation of the functions of the gut-brain-functions involved in central regulation of central dopaminergic functions and stress responses.
In conclusion, the results of the presented pilot experiments reveal that fumaric acid as well its hydrolysable esters are stress response modulating agents and suggest that their daily intake could as well be useful for prevention of mental health problems accompanying almost all chronic diseases and illnesses. They also reconfirm that bioactive constituents other than fumarates are also involved in anxiolytic like efficacy of the tested