Withania somnifera (L.) Dunal (W. somnifera; Solanaceae), popularly known as 'Ashwagandha' in Sanskrit and as 'Indian ginseng', in Ayurveda (Kulkarni and Dhir, 2008; Puri, 2003). It is a green woody shrub of 200-800 cm height, found throughout the drier parts of South East Asia including India, Bangladesh, Sri-Lanka, Nepal and Pakistan, and different other parts of Australia, Africa and America (Hepper, 1991; Ilayperuma et al., 2002; Mabberley, 2008; Mirjalili et al., 2009). In India, it is widely distributed in the provinces of Madhya Pradesh, Uttar Pradesh, Punjab Gujarat and Rajasthan (Kulkarni and Dhir, 2008). It is an important Rasayana herb used in the traditional system of medicine, for more than 2500years (Bhattacharya and Muruganandam, 2003). In Ayurvedic preparations, various parts of the plant have been used to treat variety of ailments (Kulkarni and Dhir, 2008), however roots of the plant is used to prepare tonic which promote longevity, revitalizing the body, arresting the aging process and augmenting defense against infectious diseases (Bhattacharya and Muruganandam, 2003; Singh et al., 2008). W. somnifera also participate as an important ingredient in many Ayurvedic formulations, which are currently commercialized in India and other countries. Ayurvedic formulations containing W. somnifera prescribed as analgesic for a variety of musculoskeletal disorders (arthritis and rheumatism), certain forms of hypertension, for stimulating sexual impulses and increases sperm counts, in gynecological practice for vaginitis and during pregnancy for breast development (Mishra and Singh, 2005; Puri, 2003). Since ancient times W. somnifera has been considered for persons of both sexes, of all ages and at all stages of their lives as nerve tonic, aphrodisiac, adaptogen, antirheumatic agent, astringent and memory enhancer (Bhattacharya and Muruganandam, 2003; Puri, 2003). Many pharmacological studies have been carried out to describe multiple biological properties of W. somnifera and outcomes obtained from these studies indicate that it is also useful to treat bronchitis, asthma, ulcer, cancer, emaciation, insomnia, and senile dementia (Kulkarni and Dhir, 2008; Mishra and Singh, 2005). W. somnifera also having other multipurpose medicinal uses which are supported by different preclinical and clinical trials includes for antidiabetic, immunomodulatory, hemopoietic, neurological inflammatory disorders and Parkinson’s disease, additionally it is also useful as antioxidant, abortifacient, antibiotic, aphrodisiac, deobstruent, diuretic and sedative (Bone, 1996; Kulkarni and Dhir, 2008; Mishra and Singh, 2005). Its chemopreventive properties make it a potentially useful adjunct for patients receiving radiation and chemotherapy (Devi et al., 1992).
The major biochemical constituents of W. somnifera are withanolides (steroidal lactones with ergostane skeleton). The withanolides have C28 steroidal nucleus with C9 side chain, having a six membered lactone ring. Withanolides are highly oxygenated phytochemicals, and the oxidation at various sites of skeleton is responsible for the structural variations in different classes of withanolides (Choudhary et al., 2013; Kulkarni and Dhir, 2008). In addition, different classes of withanosides, glycowithanolides, sitoindosides, alkaloids, saponins, amino acids, phenolic compounds, flavonoids and many other secondary bioactive metabolites of the plant with broad-spectrum therapeutic activity were isolated and characterized (Elsakka et al., 1990; Mishra and Singh, 2005). Much of ashwaganda’s pharmacological activity has been attributed to two main constituents such as withaferin-A and withanolide-D (Mirjalili et al., 2009).
Extensive toxicological studies and the data obtained from various clinical research works on W. somnifera was demonstrated that the plant is nontoxic in wide range of practical doses and it can be assumed that the doses in which its preparations are indicated in humans are expected to be very safe. As of today, no herb-herb or herb–drug interactions has been reported in the literature with W. somnifera (Kulkarni and Dhir, 2008; Prabu et al., 2013; Sharada et al., 1993). The side effects and the long-term safety of W. somnifera are still under construction. However, large doses of W. somnifera might cause stomach upset, diarrhea, and vomiting. Based on limited human research it was reported that W. somnifera may cause sedation, possible life-threatening respiratory depression, decrease blood pressure and cause abnormal heart rhythms (Gardner and McGuffin, 2013; Puri, 2003).
Information now available on chemistry and pharmacology of diverse types of W. somnifera extracts obtained from different parts of the plant have been reviewed which strongly suggest that appropriate combinations with other plants could be used for prevention and cure of diverse disorders and chronic diseases. In this chapter, we mainly focus on earlier and recent advancements on chemistry and pharmacological aspects of W. somnifera.
The major chemical constituents of W. somnifera are withanolides, a group of naturally occurring C28 steroidal lactones with ergostane-based skeleton. Withanolides are highly oxygenated phytoconstituents, and the oxidation at various sites of skeleton is responsible for the structural variations in different classes of withanolides. Withanolides possess a wide range of biological activities (Choudhary et al., 2013). Lavie and coworkers in 1965 were the first to isolate withaferin-A from W. somnifera (Lavie et al., 1965). Even though several reports were published in the recent literature from various research groups about evaluating different pharmacological activities of W. somnifera, very few groups have reported synthetic analogs or semi-synthetic derivatives of its potential alkaloids. Most of the analogs synthesized are the derivatives of its chief constituent withaferin-A. Here we will briefly review some of the most important developments in recent years involving chemical modification of Withanolides.
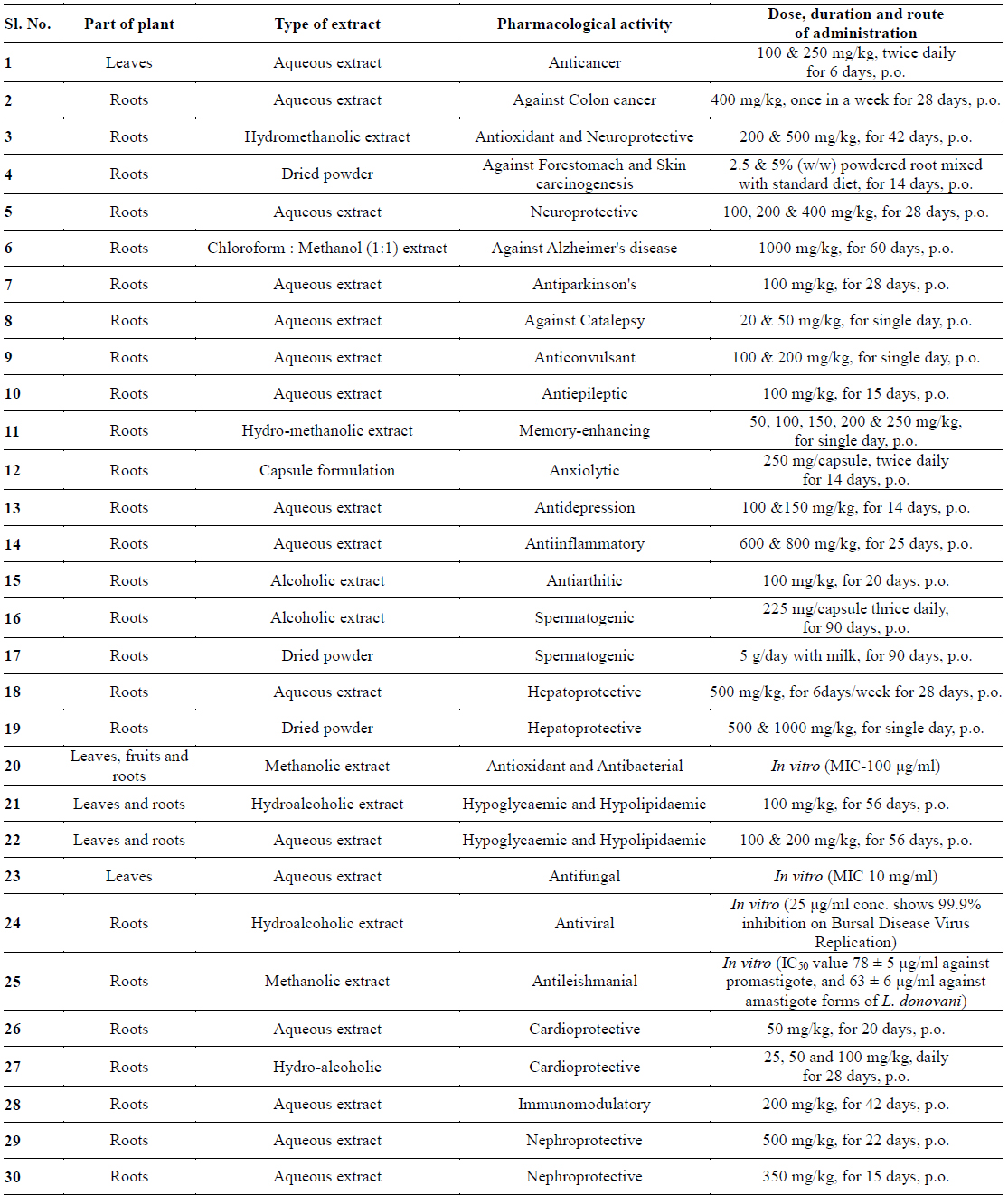
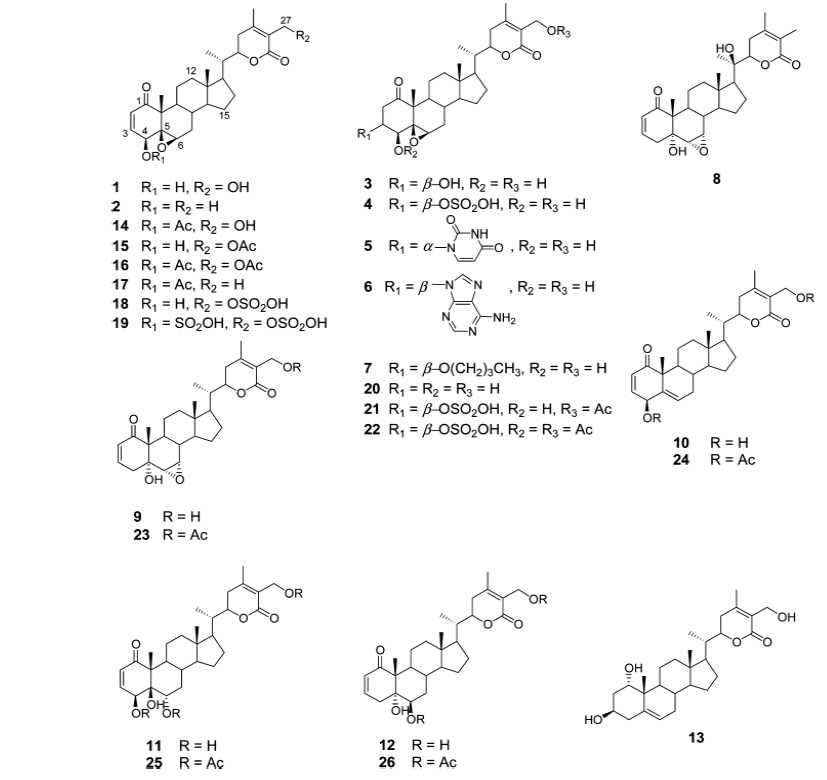
Gunatilaka group reported the synthesis and cytotoxic profile of various derivatives of withaferin-A (Wijeratne et al., 2014). They synthesized a total of 36 analogs by a variety of chemistry and compared their cytotoxicity to cytoprotective heat-shock-inducing activity to that produced by withaferin-A. By analyzing structure activity relationship for this structurally diverse group of compounds, they have found that ring aenone of withaferin-A is essential for its bioactivity. Acetylation of hydroxyl group at the 27th position leads to the loss of potency. Their detailed studies demonstrate that the basic withanolide skeleton can be modified to selectively enhance heat shocking inducing activity (Figs. 1 and 2).
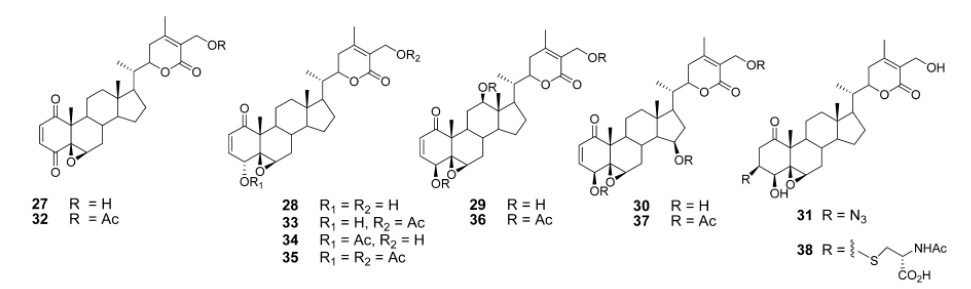
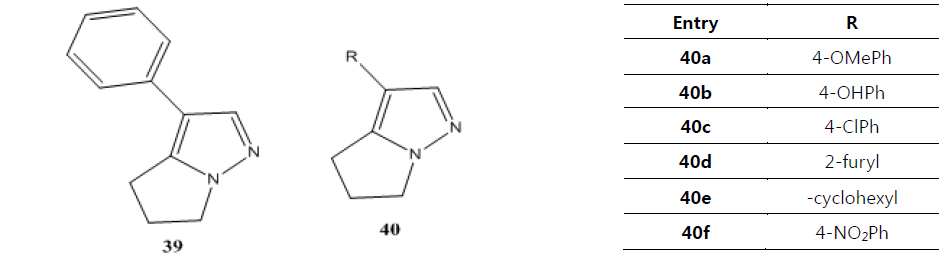
Namboothiri group has reported the total synthesis of three pyrazole containing withasomnine based alkaloids (Varma et al., 2013). Starting with 4-nitro-1-butanol and commercially available aldehydes, they synthesized these molecules in overall five steps. A wide variety of methods has been reported in the earlier literature but these methods involve complex chemistry and lower yields. The work of Namboothiri group is more efficient in terms of its reaction conditions, yields and availability of starting chemicals. The key step in these syntheses is a 1,3-dipolar cycloaddition of α-bromopropyl nitroalkenes with commercially available TMSCHN2 (Fig. 3).
With this novel method, they were able to accomplish the total synthesis of three withasomnine natural products (39 and 40a-b), and three other non-natural analogs (40c-f) (Fig. 4).
Aubé and coworkers reported a series of semisynthetic analogs of withalongolide-A, which is a C-19 hydroxylated analog of withaferin-A (Motiwala et al., 2013). These semisynthetic derivatives are found to be more cytotoxic than the parent molecule against a variety of cell lines (Fig. 5).
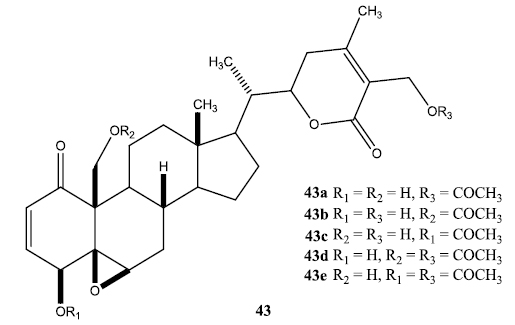
A series of aliphatic esters (43a-e) were synthesized by slight modification of standard acylation procedure with acetic anhydride. These researchers were able to isolate both mono and diacetate derivatives from one-pot reactions (Fig. 6).
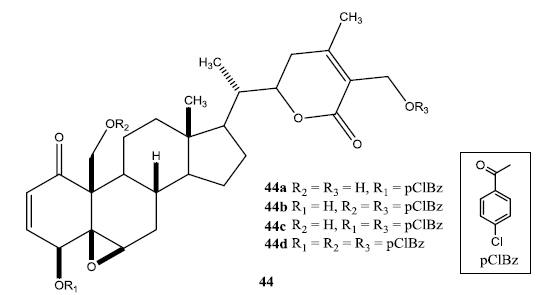
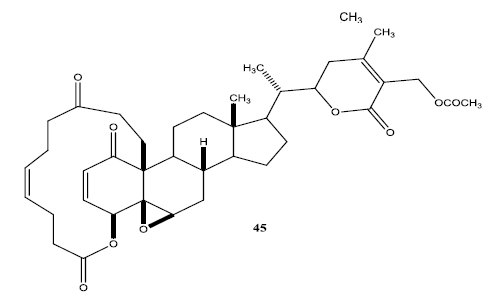
In a second set of compounds they developed benzoyl esters (44a-d) by reaction of withalongolide-A with p-chlorobenzoylchloride (Fig. 7). Another interesting molecule developed was a steroidal macrocycle, compound (45). This 14-membered macrocycle with E-configuration was obtained by bis-acylation of withalongolide-A monoacetate with 4-pentenoic anhydride followed by ring closing metathesis using Grubb’s II catalyst (Fig. 8).
All these synthesize d molecules were tested for cytotoxic properties against four different cell lines. Some of these semi synthetic derivatives are found to be more potent than the parent natural molecules.
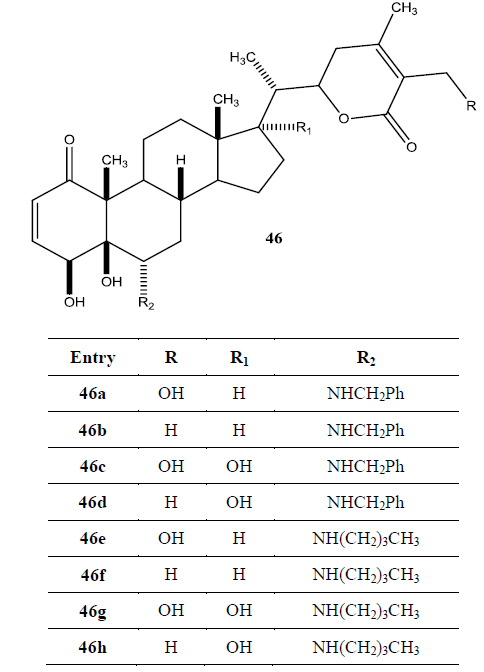
Recently Darokar group developed a series of derivatives with specific modifications at carbons 5, 6 and 7 of ring B (Joshi et al., 2014). In addition, they selectively converted the epoxide group to a thiirane, an amino alcohol and alcohol group, and all these derivatives were evaluated against four different cancer cell lines (Fig. 9).
These researchers have successfully demonstrated that a wide variety of chemistry can be applied to withanolides, and these chemical modifications have led to a decrease in cytotoxic potency.
The reports presented here firmly establish the robust nature of withaferin-A, that can be modified chemically leading to a wide variety of biologically active novel molecules. These various structural analogs of withanolides help us understand structure-activity relationships among these varied structures. Whether these novel molecules will hold any potential for the treatment of diseases other than cancer, need to be studied.
Anticancer activity
Numerous studies published over the last two decades indicate that W. somnifera has unique characteristics to suppress various types of cancer and it has been used as Ayurvedic remedy for the treatment of various types of cancer over two thousand years. Ashwagandha possesses anticancer properties against prostate, colon, lung, breast, leukemia, pancreatic, renal, head and neck cancer cells of humans (Nema et al., 2013; Patel et al., 2013; Singh et al., 2011; Yadav et al., 2010), forestomach and skin cancer cells in mice (Padmavathi et al., 2005). Recently the anticancerous potential of W. somnifera and its bioactive withanolides has been extensively studied by several research groups all around the world, which have discovered diverse mechanisms such as cytotoxicity, cell differentiation induction, cancer chemoprevention, cyclooxygenase-2 (COX-2) inhibition and a potential to inhibit the enzyme quinine reductase. These withanolides are highly oxygenated natural bioactive constituents which are responsible for ashwagandha’s biological properties including antitumor activity (Mulabagal et al., 2009; Patel et al., 2013).
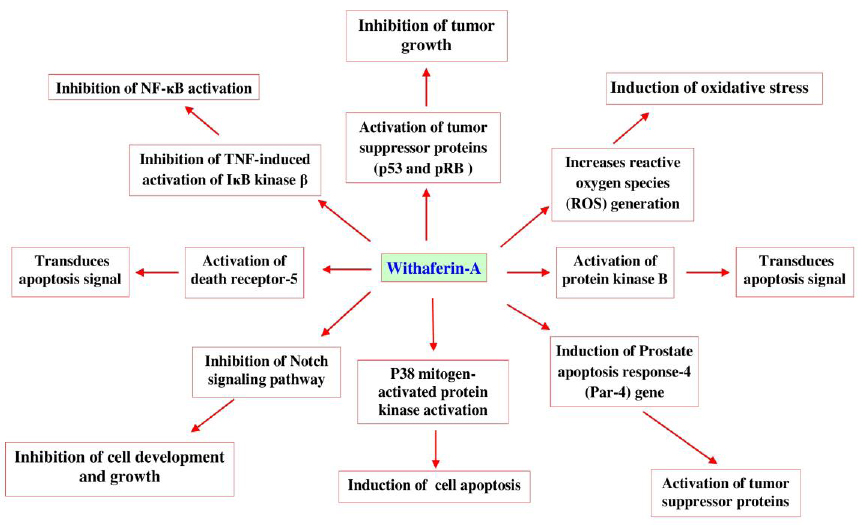
Withaferin-A, is a potent inhibitor of angiogenesis and thus protective in certain types of cancers (Patel et al., 2013). Recent investigation identifies that W. somnifera enriched withaferin-A induces apoptosis through mechanisms (Fig. 10) such as, inhibiting the activation of nuclear factor kappa-B (NF-κB) by preventing the TNF-induced activation of IκB kinase β via a thioalkylation sensitive redox mechanism (Oh and Kwon, 2009), activation of tumor suppressor proteins such as p53 and pRB (Wadhwa et al., 2013). It increases reactive oxygen species (ROS) generation, Par-4 induction and p38 MAP kinase activation to induce programmed cell death (Patel et al., 2013). It also inhibits the Notch signaling pathway and NF-κB activation, induces Akt inactivation, death receptor 5 up-regulation and down-regulation of cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein (Oh and Kwon, 2009; Patel et al., 2013; Um et al., 2012).
In a separate study on human cancer cells, the active withaferin-A from W. somnifera exhibits its cytotoxicity through Par-4 induction, through inhibition of chymotrypsin like activity of proteasome, and by covalently modifying the cysteine residue of intermediate filament protein vimentin. In addition, withaferin-A also induces actin microfilament aggregation by targeting Annexin-II (Yang et al., 2013; Yu et al., 2010). On prostate cancer cells, withaferin-A arrests G2/M phase cell cycle and prevents mitosis by up regulation of phosphorylated Wee-1, phosphorylated histone H3, p21, and Aurora B targets. On the other hand, down regulation of cyclins (A2, B1, and E2) and a reduction in phosphorylated cyclin-dependent kinase (Cdc2) (Tyr15) were observed in in vitro studies and this suggests that activation of Cdc2 leads to arrest in the M phase of cell cycle, with abnormal duplication and initiation of mitotic catastrophe that result in cell death (Roy et al., 2013). Withaferin-A also possesses its apoptotic action on human colon cancer cells through inhibition of Notch-1 signaling pathway and down regulating pro-survival pathways, such as Akt/NF-κB/Bcl-2, in three colon cancer cell lines (HCT-116, SW-480, and SW-620). It also induces apoptosis on colon cancer cells by other mechanisms like generating ROS (Koduru et al., 2010). In addition, it also induces apoptosis in human breast cancer cells by mechanisms, such as inhibition of cell migration/invasion through down regulation of signal transducer and activator of transcription (STAT3) activity (Patel et al., 2013). Suppression of X-linked inhibitor of apoptosis protein and cIAP-2 protein, and reduction in the survivin protein levels are also the common targets for withaferin-A to induce apoptosis on human breast cancer cells (MDA-MB-231 and MCF-7) (Hahm and Singh, 2013). In a recent study, it was shown that withaferin-A inhibits the vimentin cytoskeleton through perinuclear vimentin accumulation followed by rapid vimentin depolymerization and a concomitant induction of vimentin ser 56 phosphorylation to induce apoptosis on human breast cancer cells (Thaiparambil et al., 2013). Withaferin-A also reportedly increases ROS production due to inhibition of mitochondrial respiration resulting in apoptosis induction of breast cancer cells (MDA-MB-231 and MCF-7) (Hahm et al., 2011). An investigation with malignant pleural mesothelioma (MPM) cells, indicates that withaferin-A inhibited growth of the MPM cells both in murine and human by decreasing the chymotryptic activity of the proteasome that results in elevation of ubiquitinated protein levels and pro-apoptotic proteasome target proteins (p21, Bax, IkBα). The apoptosis of MPM cells was induced by activation of pro-apoptotic p38 stress activated protein kinase and caspase-3, elevated levels of pro-apoptotic Bax protein and cleavage of poly-(ADP-ribose)-polymerase (Yang et al., 2012). Withaferin-A also causes cytotoxicity in human renal carcinoma Caki cells, where studies show that it inhibits interleukin (IL)-6 induced phosphorylation of STAT3 via Tyr705 residue that resulted in reduction of Janus-activated kinase 2 activities. Withaferin-A also down-regulates the expression of STAT3 regulated genes such as Bcl-xL, Bcl-2, cyclin D1 and surviving which leads to cell apoptosis (Um et al., 2012). In pancreatic cancer cell lines (Panc-1, MiaPaCa2 and BxPc3), withaferin A inhibits Hsp90 chaperone activity through an ATP independent mechanism, resulting in Hsp90 client protein degradation thus acting against pancreatic cancer (Yu et al., 2010). In a variety studies, it was observed that W. somnifera significantly changed the levels of leucocytes, lymphocytes, neutrophils and immunoglobulins in experimental colon cancer in mice induced by azoxymethane (Muralikrishnan et al., 2010a). It was also observed that W. somnifera decreased the activities enzymes such as isocitrate dehydrogenase, succinate dehydrogenase, malate dehydrogenase and alpha-keto glutarate dehydrogenase in colon cancer bearing animals, leading to lack of nutrition of cancerous cells resulting in cell death (Muralikrishnan et al., 2010b). In high concentration, water extract of ashwagandha leaves induces apoptosis, cytotoxicity and cell death of human (YKG1, A172, U118MG) and rat (C6) glioma cell lines (Kataria et al., 2011). Although both, withaferin-A and withanolides are extracted from W. somnifera, withaferin-A is the most potent anticancer compound than withanolides (Kapoor, 2014). The results of the various findings have discovered that, W. somnifera and its chemical ingredients are effective in the prevention and treatment of several kinds of cancers including colon cancer, lung cancer, blood cancer, skin cancer, breast cancer, renal cancer, fibrosarcoma, prostate cancer and pancreatic cancer.
Neuroprotective activity
Preclinical research and clinical trials support the use of W. somnifera for the treatment of neurological conditions such as anxiety, depression, cognitive disorders, senile dementia and neurodegenerative disorders (Alzheimer’s and Parkinson’s diseases). Earlier, it was reported that the neuroprotective activity of W. somnifera root extract could be because of presence of glycowithanolides and their ability to inhibit lipid peroxidation because of their antioxidant actions. In addition, withanolides and sitoindosides (VII-X) also augment catalase and glutathione peroxidase activities in rat frontal cortex and striatum. W. somnifera was also found to improve the cognitive capabilities of the brain by increasing the cortical muscarinic acetylcholine capacity in lateral septum and frontal cortex, which suggest their capacity to affect events in the cortical cholinergic-signal transduction cascade (Schliebs et al., 1997). The pharmacological studies suggested that W. somnifera improves the athletic performance via increasing the hemoglobin count and red blood cell count, which leads to an increase in the capacity of blood to transport oxygen at a greater capacity to the peripheral system (Shenoy et al., 2012). It has been shown that W. somnifera improves endurance performance in healthy individuals at a moderate intensity of 65% VO2 max (Sandhu, 2010). W. somnifera which is rich in proteins, amino acids (glycine, alanine, tyrosine, aspartic acid, tryptophan, glutamic acid, cysteine, etc.), starch, reducing sugars, alkaloids, steroidal lactones possess an amazing nutritional value and acts as a tonic, stimulant and energy rejuvenator. Studies also revealed that, phenolic compounds present in the root of W. somnifera play an important role on overall antioxidant activities of the plant (Bhatnagar et al., 2009).
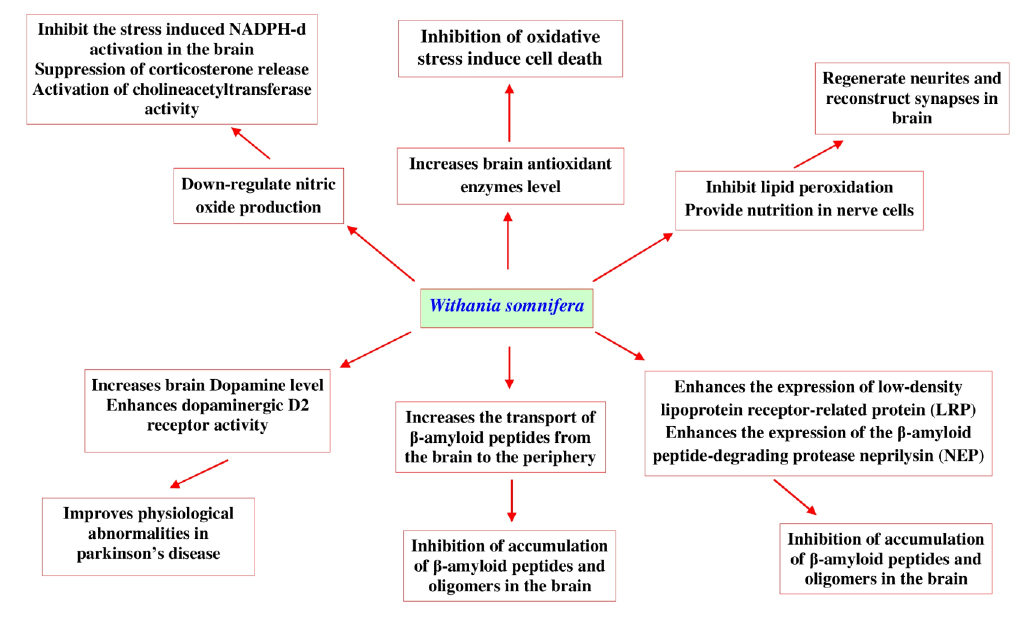
Recent research developments in this area show different mechanisms (Fig. 11) that help us understand the neuroprotective activity of W. somnifera and its bioactive withanolides.
One such report has revealed that, W. somnifera root extract down-regulates nitric oxide production that significantly inhibits the stress induced NADPH-diaphorase activation in the brain via suppressing corticosterone release and activating cholineacetyltransferase. This pathway is thought to be the main mechanism underlying the neuroprotective effects of W. somnifera (Bhatnagar et al., 2009). In another study W. somnifera root extract significantly increases the levels of brain antioxidant enzymes (superoxide dismutase (SOD), chloramphenicol acetyltransferase, glutathione, and glutathione S-transferase) and total proteins to protect the brain when animal was exposed to lead nitrate (Sharma et al., 2011). Another report also revealed that withanolide-A isolated from roots of W. somnifera at a dose 10 μmol kg-1 could regenerate neurites and reconstruct synapses in severely damaged neurons which has a huge potential in the treatment of diabetic neuropathy (Kuboyama et al., 2005). There is a scarcity of experimental data on the potential neuroprotective effects of W. somnifera against β-amyloid induced neuropathogenesis. However, in vitro studies with human neuronal SK-N-MC cell lines show that W. somnifera reverses the toxic effects when cells are intoxicated with β-amyloid (1-42) and HIV-1Ba-L (clade B) infection (Kurapati et al., 2013). Computational docking evidences reveal that withanolide-A inhibits brain acetyl cholinesterase, which could be a therapeutic alternative for the treatment of neurodegenerative Alzheimer’s disease (Grover et al., 2012). The root of W. somnifera enriched with withanolides and withanosides reversed behavioral deficits, plaque pathology, accumulation of β-amyloid peptides and oligomers in the brains of middle-aged and old amyloid precursor protein/the presenilins Alzheimer's disease transgenic mice, results of this study indicates that W. somnifera increases the transport of β-amyloid peptides from the brain to the periphery. It also enhances the expression of low-density lipoprotein receptor-related protein in brain microvessels and the β-amyloid peptide-degrading protease neprilysin (Sehgal et al., 2012). W. somnifera and its bioactive withanolides are also effective in treating Parkinson’s disease, where it was revealed that its root extract enhances brain dopamine level in Parkinson’s animals and also improves physiological abnormalities seen in Parkinson’s disease. W. somnifera improves the condition of lipidperoxidation, reduced glutathione content, activities of glutathione-S-transferase, glutathione reductase, glutathione peroxidase, superoxide dismutase and catalase, catecholamine content, dopaminergic D2 receptor binding affinity and tyrosine hydroxylase expression (RajaSankar et al., 2009). Supplement with L-Dopa W. somnifera were also effective in inhibiting haloperidol-induced catalepsy in mice (Girdhari et al., 2009). Randomized controlled clinical trial with W. somnifera root powder (3 g/day) also shows effectiveness in treating Parkinson’s disease in four weeks treatment. The results of the various findings described that, W. somnifera and its chemical ingredients are effective in prevention of neurodegenerative disorders and protect neurons from oxidative damages.
Antiepileptic activity
W. somnifera is traditionally used for the treatment of epilepsy and seizures. Various in vitro and in vivo preclinical studies have provided enough evidence for the use of W. somnifera against various types of epilepsy. In general, studies with rodent models show that W. somnifera and its bioactive withanolides are effective in reducing seizures through various mechanisms. One such mechanism involved the Gama amino butyric acid (GABA)A receptor modulation in brain, where sub-effective dose of W. somnifera (50 mg/kg), with a sub-protective dose of either GABA (25 mg/kg) or Diazepm (0.5 mg/kg) increases the seizure threshold in brain (Kulkarni et al., 2008). In another study, it was demonstrated that W. somnifera root extract and withanolide-A were capable of restoring spatial memory deficit by inhibiting oxidative stress induced alteration in glutamergic neurotransmission, where W. somnifera reduces the expression of N-metyl-D-aspartate (NMDA) receptor, which is responsible for spatial memory loss in epileptic rats (Soman et al., 2012). Leaf extracts of W. somnifera were also showing its protective action against glutamate induced toxicity in human neuroblastoma (IMR-32) cells, by inhibiting over expression of stress protein 70 kilodalton heat shock proteins (Kataria et al., 2012). In another study it was found that W. somnifera root extract and withanolide-A regulate the expression and function of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor and glutamate levels in brain dopaminergic nervous system and results are attributed to improvement in motor learning in pilocarpine-induced temporal lobe epilepsy model (Soman et al., 2013). These findings reveal that W. somnifera and its bioactive withanolides have anti convulsant potential and are useful in treating various types of epilepsy.
Antidepression and antianxiety activity
The roots of W. somnifera are used extensively in Ayurveda for the treatment of anxiety and depression. Earlier it was reported that, anxiolytic-antidepressant potential of W. somnifera and its glycowithanolides (Bhattacharya et al., 2000). Recent study reports also support the use of W. somnifera for depression and anxiety disorders. In a very recent study, it is found that W. somnifera at 40 mg/kg significantly reduces the depression in various experimental models (Jayanthi et al., 2012). Clinical trials with healthy volunteers also revealed that aqueous extracts of W. somnifera improve the psychomotor performances in anxiety and depression (Pingali et al., 2014). It was assumed that W. somnifera reduces the production of nitric oxide in the brain tissues, resulting in its anxiolytic activity (Khan and Ghosh, 2011; Maity et al., 2011). Study findings explained that W. somnifera and its bioactive withanolides possesses antidepression and antianxiety potential and are useful in treating various types of mental disorders.
Antiinflammatory and antiarthritic activity
W. somnifera exhibits potent antiarthritic and antiinflammatory activities. Antiinflammatory activity has been characteristic to biologically active steroids, of which withaferin-A is a major component. Recent studies revealed that W. somnifera at dose levels 600 & 800 mg/kg significantly decreased the severity of arthritis by effectively suppressing the inflammatory mediators and improving the functional recovery of motor activity in experimental animals (Gupta and Singh, 2014). Roots of W. somnifera and with anolides are also effective in treating arthritic inflammation, inflammation in cystic fibrosis and irritable bowel syndrome, through various mechanisms such as inhibiting NF-κB activation, inhibition of COX-2 generation, inhibition of endothelial cell protein C receptor through antioxidant effect and cytokines release, thus in turn causes depletion of inflammatory mediators (Ku et al., 2014; Mulabagal et al., 2009; Oh and Kwon, 2009). W. somnifera and its bioactive withaferin-A down regulate the production of inflammatory mediators like prostaglandins, histamine, interleukins and cytokines (Gupta and Singh, 2014; Paval et al., 2009). Withaferin-A has been shown to stimulate differentiation and growth of osteoblasts in menopausal osteoporosis and by bone injury, via increased expression of osteoblast-specific transcription factor and mineralizing genes (Khedgikar et al., 2013). Through all these mechanisms W. somnifera shows its antiinflammatory and antiarthritic activity, which makes it useful for the treatment of various inflammatory disorders. W. somnifera is also shown to possess analgesic activity in several rodent models and thus preferred for various pain management therapies (Sabina et al., 2009; Shahriar et al., 2014).
Spermatogenic activity
Several investigators reports have suggested that W. somnifera is beneficial in the treatment of male infertility. Experimental evidences have shown that treatment with W. somnifera induced testicular development and spermatogenesis in immature Wistar rats by directly affecting the seminiferous tubules, improved pro-sexual behavior of sexually sluggish mice, and increased testicular daily sperm production and serum testosterone level. W. somnifera, also counteract the oxidative damage to the sperm and reactive oxygen species associated with abnormal sperm parameters leading to infertility (Ambiye et al., 2013). In recent years, it has been well documented that W. somnifera improves semen quality by effectively reducing oxidative stress and improving reproductive hormone levels in infertile male patients (Ahmad et al., 2010; Shukla et al., 2011). In clinical trials with infertile male patients, W. somnifera repairs the altered concentrations of lactate, alanine, citrate, glycerylphosphorylcholine, histidine, and phenylalanine in seminal plasma, and it recovers the quality of semen of post-treated compared to pre-treated men, in addition to inducing spermatogenesis in infertile male patients (Ambiye et al., 2013; Gupta et al., 2013). W. somnifera boosts enzymatic activity of metabolic pathways and energy metabolism. These evidences support the use of W. somnifera for the treatment of male infertility.
Hepatoprotective activity
Various studies were performed to evaluate the hepatoprotective potential of W. somnifera. As it is used for various ailments, the hepatoprotective activity was also considered for its effective use. Investigations have given numerous evidences, where W. somnifera at a dose 500 mg/kg significantly reduces the elevated biomarkers (aspartate aminotransferase, alanine transaminase, alkaline phosphatase, and Bilirubin) in experimental animals when exposed to hepatotoxic dose of paracetamol. It significantly reduces the lipid peroxidation, enhances glutathione content, catalase, glutathione reductase and glutathione peroxidase activity in liver (Malik et al., 2013; Sabina et al., 2013). In another study, W. somnifera has shown its hepatoprotective activity against gamma radiation induced toxicity in rodents, where 100 mg/kg dose of W. somnifera significantly decreases hepatic serum enzymes, levels of malondialdehyde, total nitrate/nitrite NO(x) and also heme oxygenase activity in liver. Serum antioxidant enzymes, including SOD and glutathione peroxidase in hepatic tissues were elevated (Hosny and Farouk, 2012). These study findings support the use of W. somnifera for various hepatic disorders.
Antimicrobial activity
The leaves and roots of W. somnifera have been shown to exhibit antimicrobial activity in recent studies. Leaf extracts at concentrations 6.25 mg/ml and 12.5 mg/ml inhibited the growth of five Gram-negative pathogenic bacteria (Escherichia coli, Salmonella typhi, Citrobacter freundii, Pseudomonas aeruginosa and Klebsiella pneumonia) (Alam et al., 2012). Isolated flavonoids and alkaloids from W. somnifera show growth inhibitory activity against Enterobacter aerogens, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Raoultella planticola and Agrobacterium tumefaciens at concentration 0.039 mg/ml (Singh and Kumar, 2011; Singh and Kumar, 2012). In a separate study, crude extract of leaves of W. somnifera was tested against clinical pathogens Staphylococcus aureus, roteus mirabilis Streptococcus mutans, Streptococcus sobrinus and Salmonella paratyphi B, where it was found that 100 μl of extracts (100 mg/ml) was able to inhibit the growth of all the pathogenic bacteria (Al-Ani et al., 2013; Pandit et al., 2013). The antimicrobial potency of W. somnifera was thought to be due to its antioxidant properties (Alam et al., 2012). The ascorbic acid, anthocyanin and polyphenols found in W. somnifera leaves could inhibit microorganisms via iron deprivation or hydrogen bonding with vital proteins such as microbial enzymes (Scalbert, 1991). W. somnifera reported to exhibit antibacterial activities and it shows its activity against both Gram-positive and Gram-negative pathogenic bacteria (Singariya et al., 2012). In another study, it was argued that W. somnifera shows its bactericidal and fungicidal activity through mechanisms attributed to cytotoxicity, gene silencing and immune potentiation, where aerial extract at concentration 1.56 mg/ml shows good antimicrobial potency (Mwitari et al., 2013). It is also shown to be a moderate to active against Microsporum gypseum, Candida albicans and Cryptococcus neoformans (Mwitari et al., 2013). Withanolide D, E and F showed potent activity against PknG target in Mycobacterium tuberculosis (Santhi and Aishwarya, 2011). It has also been shown in different studies that W. somnifera have the best antimicrobial (1.5625 mg/ml), immunopotentiation (2 times IL-7 mRNA expression) and safety level (IC50 200 mg/ml). Results of these findings reveal that extracts of W. somnifera and its bioactive constituents possess great antimicrobial potential against various test pathogenic microorganisms that can be exploited for future antimicrobial drugs for treating infectious diseases and could be an alternative for chemotherapy.
Hypoglycaemic and hypolipidemic activity
W. somnifera has long been used in traditional and Ayurvedic medicine to cure diabetes and obesity. Recent studies and observations have revealed that, flavonoids found in the roots of W. somnifera were able to reduce the high blood glucose level in experimental animals. It was also shown that W. somnifera at dose 100 mg/kg significantly reduces the blood glucose and lipid levels (Rajangam, et al., 2009). In another study, powder of W. somnifera at dose 200 mg/kg significantly reduces the blood glucose level. The blood glucose lowering activity of W. somnifera is thought to be its action on pancreatic β-cells to stimulate the release of insulin. It was also found that W. somnifera and its glycowithanolides induce the transport of glucose into the cells, stimulate the release of insulin and increase the activity of GLUT transporters activity (Anwer et al., 2008; Khalili, 2009; Sarangi et al., 2013; Visavadiya and Narasimhacharya, 2007). In another study it was observed that, W. somnifera diet significantly increases plasma HDL-cholesterol levels, increases HMG-CoA reductase activity and bile acid content of liver in experimental animals (Visavadiya and Narasimhacharya, 2007). These observations support the traditional claims for the use of W. somnifera against diabetes and obesity.
Miscellaneous pharmacological activities
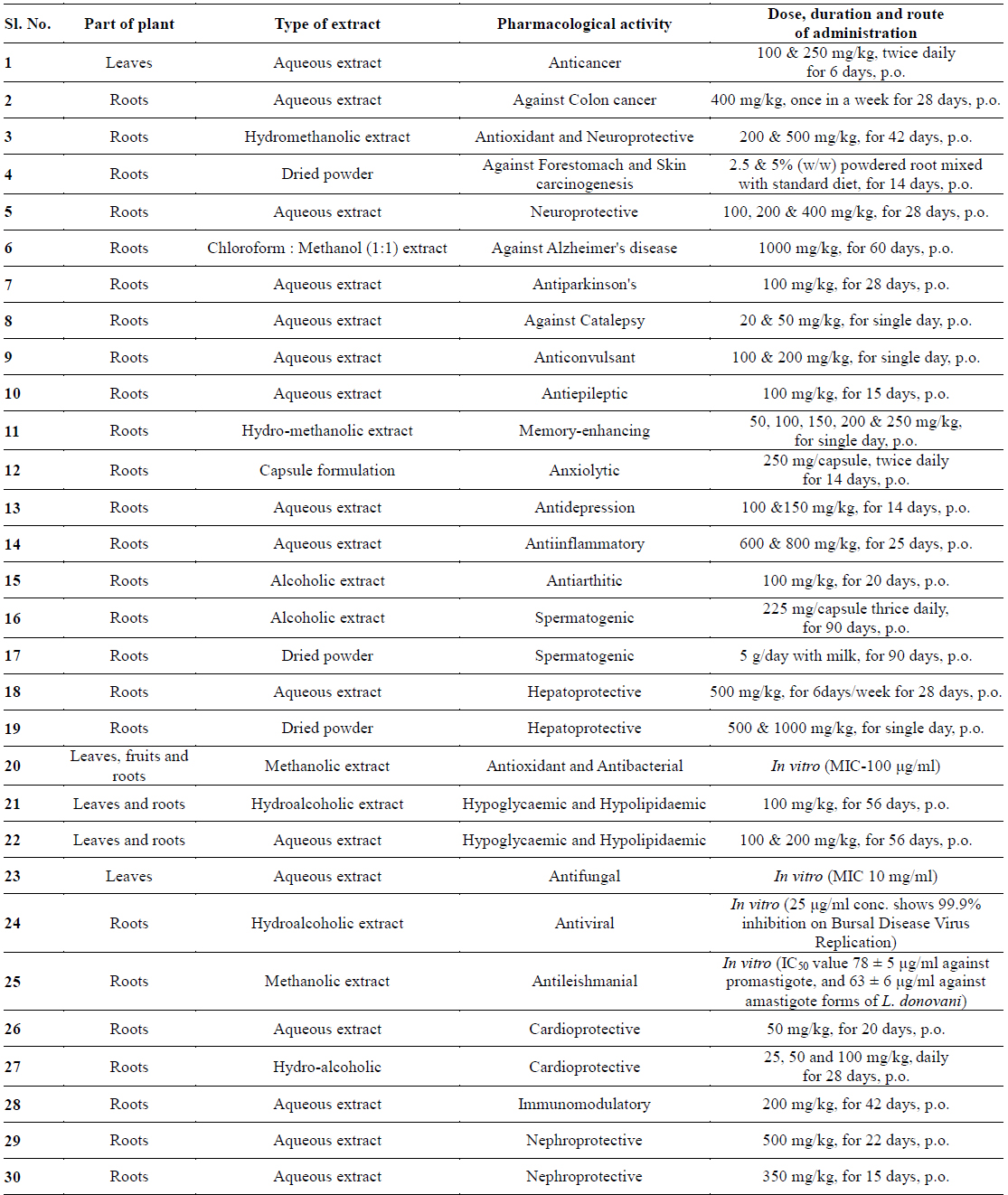
In recent years, numerous pharmacological studies were also carried out to explore other beneficial effects of W. somnifera. Further research with withaferin-A shows that having antiplatelet, anticoagulant, and profibrinolytic properties (Ku and Bae, 2014), cardioprotective activity, nephroprotective activity, immunomodulatory activity and antileishmanial activities. Details of various pharmacological activities are illustrated in Table 1.