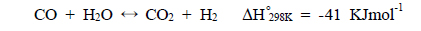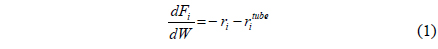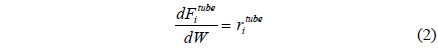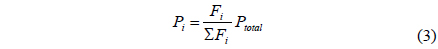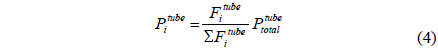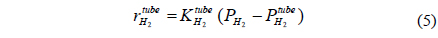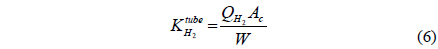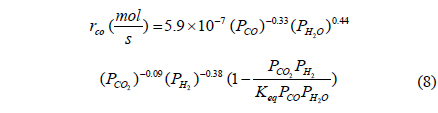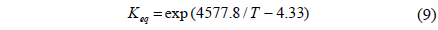이 논문은 촉매막반응기(catalytic membrane reactor)에서의 중요한 두 요소인 수소선택도와 수소투과량 및 Ar sweep 유량과 압력이 수성가스전이반응의 성능에 미치는 영향에 대하여 1차원 반응기모델과 반응속도식에 근거한 연구결과를 나타내고 있다. 연소전 이산화탄소 포집의 한 방법으로서, 촉매막반응기를 사용하여 원통부분에서는 고압/고농도의 이산화탄소를 관부분에서는 고순도의 수소를 동시에 얻을 수 있는지에 대한 가능성을 검토하였다. 또한, 고농도의 이산화탄소와 고순도의 수소를 동시에 얻기 위해 필요한 수소투과량, 수소선택도, Ar sweep 유량 및 압력에 대한 지침을 나타내었다. 그 결과 1 × 10-8 molm-2s-1Pa-1의 수소투과량과 10000의 수소선택도를 가진 막을 장착한 촉매막반응기에서는 8 atm의 압력과 6.7 × 10-4 mols-1의 Ar sweep 유량의 조건하에서 약 90%의 농도를 가진 이산화탄소와 100%의 순도를 가진 수소가 동시에 얻어짐이 밝혀졌다.
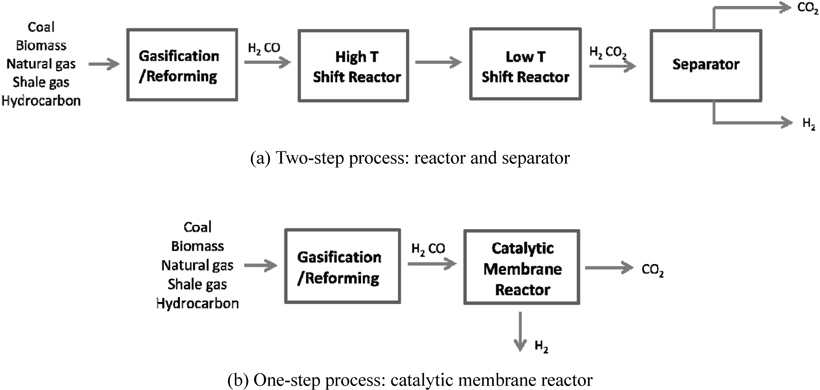
Recent issues like environmental pollutions and CO2 emissions have led to a strong demand for a clean energy such as solar energy, hydrogen, and fuel cells. However, it is very unlikely for these technologies to realize in a near future from an economical point of view and it will be inevitable to depend on current fossil fuel based energy. Therefore, many efforts have been made to reduce or capture CO2, which is considered as a green house gas causing a global warming, released from the use of current fossil fuels and three different types of CO2 capture methods, post-combustion, oxy-fuel, and pre-combustion have been extensively studied so far[1-5]. A post-combustion CO2 capture is to capture CO2 from a flue gas released from burning fossil fuels and a commercially available amine absorption technology can be used for this method, but it is very expensive and energyintensive process. In addition, an additional compression step is required for a subsequent CO2 sequestration because the pressure of a captured CO2 is relatively low. Oxy-fuel is a new concept of using pure O2 instead of air so that a flue gas after gasification contains only CO2 and steam. In oxy-fuel, a concentrated CO2 can be obtained in a flue gas by removing steam in a cooler, but this process requires an additional air separation unit (ASU) to separate O2 from air to provide a pure O2 to a gasification/reforming process. A pre-combustion CO2 capture is to separate CO2 from a stream containing mainly H2 and CO2 in order to only combust H2, a clean fuel, and the captured CO2 can be further sequestered. Figure 1(a) shows a gasification/ reforming process to produce a synthesis gas coupled with a pre-combustion CO2 capture process. Initially, different feed streams such as coal, biomass, natural gas (NG), shale gas, and hydrocarbon are fed to a gasifier or reformer to produce a synthesis gas, a H2-CO rich stream, and then undergo high temperature and low temperature shift reactors to produce H2-CO2 rich stream by a water-gas shift reaction described in reaction 1.
This stream is then separated into H2 and CO2 by a separator and H2-rich stream is combusted and CO2-rich stream is transported and further sequestered. This process is called a two-step process because a shift reactor and a separator are needed independently and separators using solvents, physical/chemical adsorbents, or membranes can be used [1]. Figure 1(b) presents a new concept of a one-step process employing a catalytic membrane reactor (CMR) combining a reactor for a water gas shift reaction and a separator for H2-CO2 separation and this process is very simple and cost-efficient because two independent units are merged onto a single unit. Moreover, a simultaneous production of a pressurized and concentrated CO2 in a retentate (a shell side of a CMR) and purified H2 in a permeate (a tube side of a CMR) can be achieved in a CMR with the use of a hydrogen separation membrane and an enhanced H2 production is expected from an equilibrium shift by continuously removing H2 during reaction (
A CMR consists of a reactor with a catalyst for reaction and a separator with a membrane for separation and has been extensively used for H2 producing processes like various reforming reactions [7-12]. For a membrane separation, there are two key factors to determine the quality of a membrane of interest, hydrogen permeance and hydrogen selectivity [13]. A hydrogen permeance(
A one-dimensional reactor model with reaction kinetics was used to model a CMR[16] using equations 1-7 with
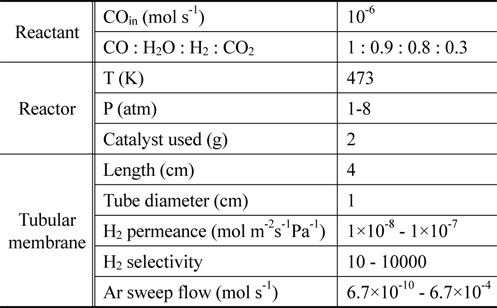
From previously reported reaction kinetics for a water gas shift reaction[17], an empirical rate equation (Equation 8 and 9) obtained from kinetic data at 473 K by Phatak et al.[18] was chosen for this study because the kinetic data were obtained using a Pt/CeO2 catalyst showing high stability in air and at high temperatures as well as sulfur tolerance. The composition of a flue gas obtained from different gasifiers such as GE Energy Radiant, Conoco-Phillips E-Gas, KBR, and Shell was used for a feed stream to a CMR and reaction conditions and membrane properties are shown in Table 1.
[Table 1.] Reaction conditions and membrane properties used in a catalytic membrane reactor
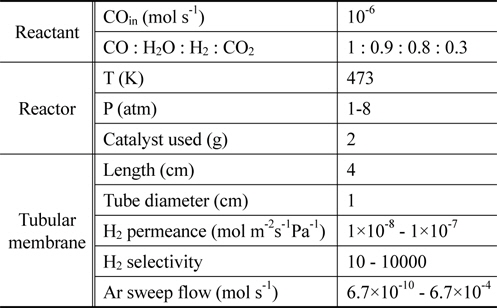
Reaction conditions and membrane properties used in a catalytic membrane reactor
3.1. Effect of an operating pressure and a hydrogen selectivity on the performance of a CMR
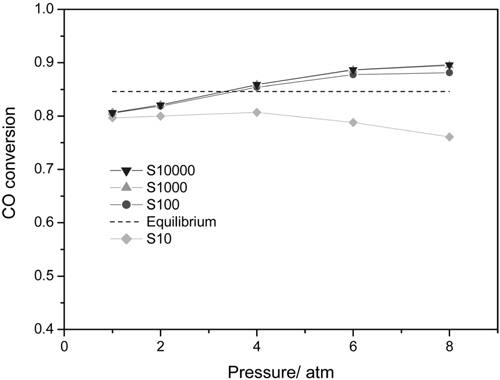
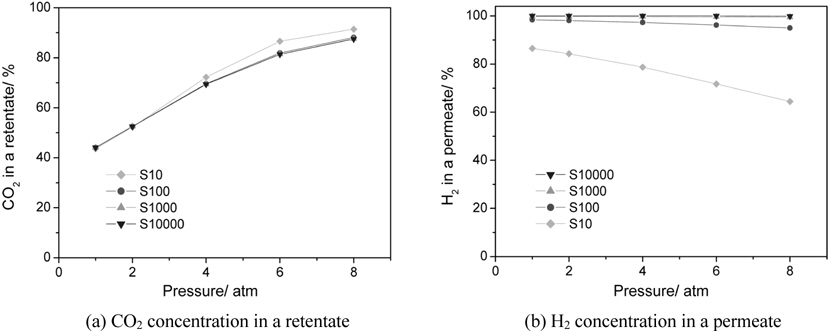
CMR studies were carried out at an operating pressure of 1-8 atm and a reaction temperature of 473 K using various membranes with a hydrogen selectivity ranging from 10 to 10000. The broad range of a hydrogen selectivity was chosen to extensively investigate the effect of a hydrogen selectivity on the performance of a CMR and the hydrogen selectivity of 10 represents a microporous membrane with a Knudsen diffusion mechanism, in which a permeance of a gas molecule is inversely proportional to the square root of its molecular weight while the hydrogen selectivity of 10000 represents a hydrogen perm-selective membrane like a palladium membrane[19,20]. Figure 2 shows the effect of an operating pressure and a hydrogen selectivity on a CO conversion at 473 K in a CMR fitted with a membrane with a hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1. An equilibrium CO conversion of 0.846 at the reaction condition (dashed line) was obtained from UniSim® Design Suite using a Gibbs reactor model and Peng-Robinson equation of state. For a membrane with a hydrogen selectivity of 10, a CO conversion slightly increased with pressure initially because of an increased driving force between a retentate and a permeate caused by increased pressure, but then declined with pressure. In addition, for all pressures studied a CO conversion was less than an equilibrium conversion of 84.6 indicating no benefit of using a CMR and it is surmised that a low hydrogen selectivity of 10 led to a permeation of a reactant, CO, through a membrane and less CO to react remained in a shell side of a CMR (retentate) resulting in a low CO conversion. However, as a hydrogen selectivity increased, a CO conversion higher than the equilibrium was obtained at a certain pressure range. For example, for a hydrogen selectivity of 100 a CO conversion increased with pressure considerably because of an increased driving for between a retentate and a permeate and then exceeded an equilibrium at a pressure of about 3.5 atm confirming the benefit of a CMR that an enhanced reactant conversion can be obtained by a shift of equilibrium by continuously removing hydrogen during reaction. Moreover, for a hydrogen selectivity of 1000 and 10000 a similar trend of increasing CO conversion with pressure was observed and a CO conversion surpassed an equilibrium one at a pressure of about 3.25 atm also confirming the benefit of a membrane reactor. One interesting thing to note is that a CO conversion increased noticeably at low pressure and then increased only slightly at high pressure and it is conjectured that an additional pressure effect of driving more hydrogen through a membrane decreased under a current hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1. In other words, it can be said that a membrane with a higher hydrogen permeance is required to fully utilize the benefit of a CMR at high pressure.
Figure 3 presents the effect of an operating pressure and a hydrogen selectivity on a CO2 concentration in a retentate (a shell side of a CMR) and H2 concentration (H2 purity) in a permeate (a tube side of a CMR) with a fixed hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1 and this is to test the feasibility of employing a CMR, a simple and less expensive process, for a water gas shift reaction after a gasification/reforming process as a precombustion CO2 capture method as described in Figure 1(b). For a CO2 concentration in a retentate, it is apparent that pressure had a positive effect for all CMRs studied and a CO2 concentration close to 90% was obtained at 8 atm in a retentate side for all membranes while a CO2 concentration of about 40% was attained at 1 atm for all membranes as shown in Figure 3(a). It is believed that pressure was a strong driving force for a H2 penetration through a membrane resulting in less H2 and more CO2 in a retentate. Interestingly, the highest CO2 concentration in a retentate was achieved in a membrane with a hydrogen selectivity of 10 and it is conjectured that reactants such as CO and H2O as well as H2 could easily pass through a membrane with pressure due to a low hydrogen selectivity and this resulted in a relatively increased CO2 concentration in a retentate. On the other hand, the effect of an operating pressure and a hydrogen selectivity on a H2 concentration in a permeate is shown in Figure 3(b). For a membrane with a hydrogen selectivity of 10 a H2 concentration obviously decreased dramatically with pressure from 87% at 1 atm to 64% at 8 atm because pressure made other species such as CO, H2O, CO2 pass through a membrane leading to a less H2 concentration in a permeate. There was a slight decrease of a H2 concentration in a permeate with pressure for a membrane with a hydrogen selectivity of 100 (98% at 1atm and 95% at 8 atm) and little decrease for a hydrogen selectivity of 1000 and 10000 with pressure (close to 100%). From combined results presented in Figure 3(a) and 3(b), it can be concluded that a membrane with a hydrogen selectivity close to 100 is required to obtain a pressurized and concentrated CO2 close to 90% in a retentate and a purified H2 close to 95% in a permeate at the same time. In addition, these results suggest that a membrane should be very carefully chosen depending on its application and there can be no big advantages of using expensive Pd-based membranes with infinite selectivity compared to other inexpensive membranes with a hydrogen selectivity of about 100 for a precombustion CO2 capture in a CMR.
3.2. Effect of a hydrogen permeance and an operating pressure on the performance of a CMR
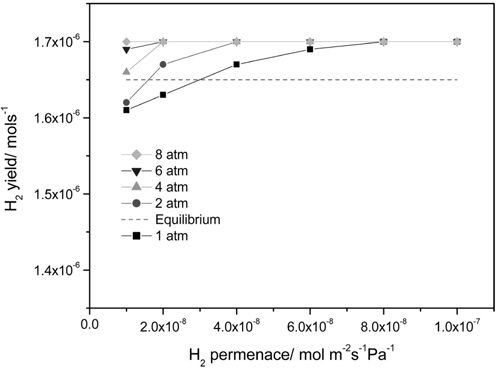
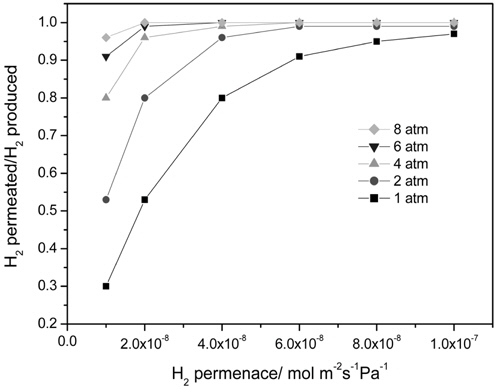
Figure 4 presents the effect of a hydrogen permeance and pressure on a H2 yield at 473 K in a CMR with a fixed hydrogen selectivity of 10000 representing Pd-based membranes. The range of a hydrogen permeance studied was 1 × 10-8 mol m-2s-1 Pa-1 to 1 × 10-7 mol m-2s-1Pa-1 and an equilibrium H2 yield of 1.65 × 10-6 mol s-1 at the reaction condition (dashed line) was obtained from UniSim® Design Suite using a Gibbs reactor model and Peng-Robinson equation of state. For an operating pressure of 1 atm, a H2 yield increased with an increasing H2 permeance and a H2 yield higher than an equilibrium one was attained at a hydrogen permeance higher than 3 × 10-8 mol m-2s-1Pa-1 due to a continuous removal of hydrogen through a membrane confirming the benefit of a CMR. A similar trend was obtained for an operating pressure of 2 atm and a H2 yield higher than an equilibrium one was attained at a hydrogen permeance higher than 1.5 × 10-8 mol m-2s-1Pa-1, which is less than the one obtained for 1 atm. In addition, a H2 yield higher than an equilibrium one was obtained for an operating pressure of 4, 6, and 8 atm irrespective of a hydrogen permeance. Apparently, a hydrogen permeance was favorable for a H2 yield and an increased pressure lowered minimum hydrogen permeance required to achieve a H2 yield higher than an equilibrium one. It is also noteworthy that a H2 yield reached a saturated yield of 1.7 ×10-6 mol s-1 for all hydrogen permeance studied in this paper possibly because most of hydrogen produced was removed from a retentate during reaction resulting in no further driving force for H2 transport through a membrane at a high hydrogen permeance. A ratio of a hydrogen permeated through a membrane and a hydrogen produced during reaction is shown in Figure 5 and obviously there was a significant difference in the ratio for 1 atm leading to a big change in a H2 yield with a hydrogen permeance while there was less difference in the ratio with higher pressure resulting in a slight change in a H2 yield. In addition, the ratio was close to 1.0 for all pressure at a hydrogen permeance of 1 × 10-7 mol m-2s-1Pa-1 and these results can explain why a saturated H2 yield was obtained at a hydrogen permeance of 1 × 10-7 mol m-2s-1Pa-1.
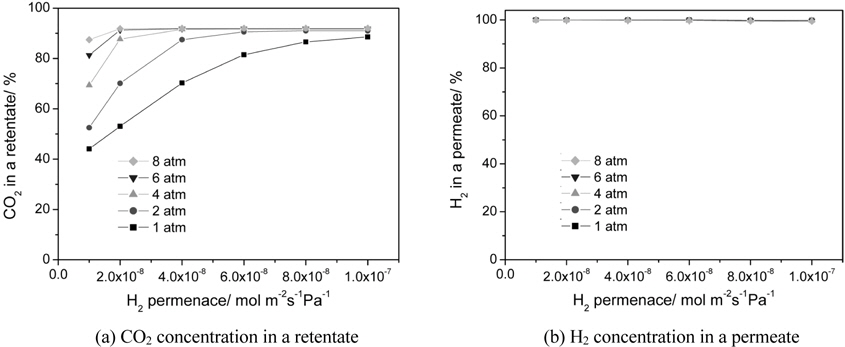
The effect of a hydrogen permeance and an operating pressure on a CO2 concentration in a retentate and a H2 concentration (H2 purity) in a permeate is shown in Figure 6(a) and 6(b), respectively. A H2 concentration in a permeate close to 100% was obtained for all hydrogen permeance studied because a membrane with a hydrogen selectivity of 10000 was used. For a CO2 concentration in a retentate, a general trend of a higher CO2 concentration with pressure was attained mainly because pressure was favorable for hydrogen permeation through a membrane. For a hydrogen permeance of 1 × 10-8 to 1 × 10-7 mol m-2s-1Pa-1, a significant increase of a CO2 concentration from 44 to 89% was observed for a pressure of 1atm with a slight increase of a CO2 concentration from 88 to 92 for a pressure of 8 atm. In other words, for a high operating pressure a CO2 concentration close to 90% was attained even at a hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1 because higher pressure led to a higher driving force for a H2 transport through a membrane.
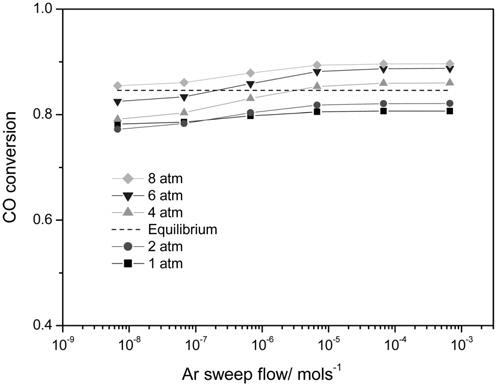
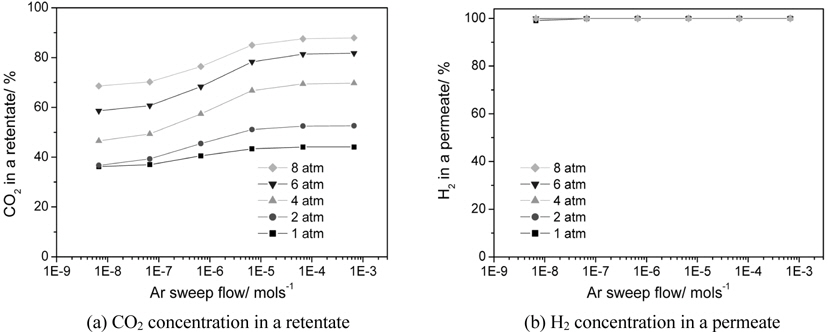
3.3. Effect of an Ar sweep flow and an operating pressure on the performance of a CMR
There are two methods to increase a hydrogen permeation through a membrane in order to fully utilize the benefits of a CMR. One is to pressurize a retentate so that more hydrogen can pass through a membrane and the other is to use an inert sweep gas in a permeate so that a hydrogen concentration difference between a retentate and a permeate increases by continuously sweeping a permeated hydrogen. Figure 7 shows the effect of two factors, an Ar sweep flow rate and an operating pressure, on a CO conversion at 473 K in a CMR with a fixed hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1 and hydrogen selectivity of 10000. The general trend of higher CO conversion with an Ar sweep flow rate was obtained reflecting the benefit of using a sweep flow in a CMR for an improved driving force for a hydrogen permeation. For an operating pressure of 1 and 2 atm, a CO conversion never exceeded an equilibrium one irrespective of an Ar sweep flow rate, but a CO conversion surpassing an equilibrium one appeared for a pressure of 4,6, and 8 atm. Respective Ar sweep flow rates of 3.5 × 10-6 and 2.0 × 10-7 mol s-1 were required to achieve a CO conversion higher than an equilibrium one for respective operating pressures of 4 and 6 atm while a CO conversion higher than an equilibrium was attained at 8 atm irrespective of an Ar sweep flow rate. The effect of an Ar sweep flow and an operating pressure on a CO2 concentration in a retentate and a H2 concentration in a permeate is shown in Figure 8(a) and 8(b). A clear trend of an increasing CO2 concentration with both an Ar sweep flow and an operating pressure was obtained, but a CO2 concentration close to 90% was obtained only with an Ar sweep flow rate of 6.7 × 10-4 mols-1 and an operating pressure of 8 atm because a membrane with a low hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1 was used in a CMR and these results confirm the need to develop a hydrogen separation membrane with a high hydrogen permeance.
A numerical simulation using a one-dimensional reactor model and reaction kinetics has been carried out for a water gas shift reaction in a catalytic membrane reactor (CMR) with a feed stream obtained from real coal gasifiers and feasibility studies of a simultaneous production of a pressurized and concentrated CO2 and a purified H2 in a CMR have been performed.
It was found that a CO conversion never surpassed the equilibrium one for all pressure studied for a membrane with a hydrogen selectivity of 10 and a CO conversion higher than the equilibrium one was obtained only for a membrane with a hydrogen selectivity of 100, 1000, 10000, reflecting the benefit of employing a CMR.
A hydrogen permeance of a membrane had a positive effect on a H2 yield in a CMR and a H2 yield higher than an equilibrium one was obtained for all pressure studied. A further analysis using a ratio of hydrogen permeated and hydrogen produced revealed that a significant H2 yield increase was observed at a low pressure because of a dramatic change of the ratio while a slight H2 yield was obtained at a high pressure due to a little change of the ratio.
A CO conversion never exceeded an equilibrium one for an operating pressure of 1 and 2 atm irrespective of Ar sweep flow rate while a minimum Ar sweep flow rate to obtain a CO conversion higher than an equilibrium one existed for an operating pressure of 4 and 6 atm. Interestingly, a CO conversion surpassing an equilibrium one was observed for all Ar sweep flow rates studied at an operating pressure of 8 atm. For a hydrogen permeance of 1 × 10-8 mol m-2s-1Pa-1and a hydrogen selectivity of 10000, a concentrated CO2 in a retentate (~90%) and a purified H2 in a permeate (~100%) was attained simultaneously only at an Ar flow rate of 6.7 × 10-4 mols-1 and an operating pressure of 8 atm.
In conclusion, these studies provide a very useful guideline for a hydrogen permeance and a hydrogen selectivity coupled with an operating pressure and an Ar sweep flow to achieve a simultaneous production of a concentrated CO2 in a retentate and a purified H2 in a permeate and show the feasibility of employing a CMR as a pre-combustion CO2 capture method.
Fi Molar flow rate of species i on a shell side of a CMR (retentate) (mol s-1) Molar flow rate of species i on a tube side of a CMR (permeate) (mol s-1) W Catalyst weight (g) ri Reaction rate equation of reaction i (mol s-1 g-1) Permeation rate equation of species i through a membrane (mol s-1 g-1) Pi Partial pressure of species i on a shell side of a CMR (retentate) (atm) Partial pressure of species i on a tube side of a CMR (permeate) (atm) Ptotal Total pressure on a shell side of a CMR (retentate) (atm) Total pressure on a tube side of a CMR (permeate) (atm) Proportional constant of (mol s-1 g-1 atm-1) QH2 Hydrogen permeance (mol m-2s-1Pa-1) Ac Cross-sectional area of a membrane (m2) αi Hydrogen selectivity of species i T Temperature (K) Keq Equilibrium Constant
CMR Catalytic Membrane Reactor