Bufonis venonum (BV) is the dried white secretions of the auricular and skin glands of the toads Bufo bufo gargarizans or Bufo melanosticus Schneider. This study was performed to evaluate the toxicity of intramuscularly- administered Bufonis venonum pharmacopuncture (BVP) and to calculate its approximate lethality through a single-dose test with Sprague-Dawley (SD) rats.
Twenty male and 20 female 6-week-old SD rats were injected intramuscularly with BVP or normal saline. The animals were divided into four groups with five female and five male rats per group: the control group injected with normal saline at 0.5 mL/animal, the low-dosage group injected with 0.125 mL/animal of BVP, the medium-dosage group injected with 0.25 mL/animal of BVP and the high-dosage group injected with 0.5 mL/animal of BVP. All injections were in the left thighs of the rats. After administration, we conducted clinical observations everyday and body weight measurements on days 3, 7 and 14 after the injection. We also carried out hematology, serum biochemistry, and histological observations on day 15 after treatment.
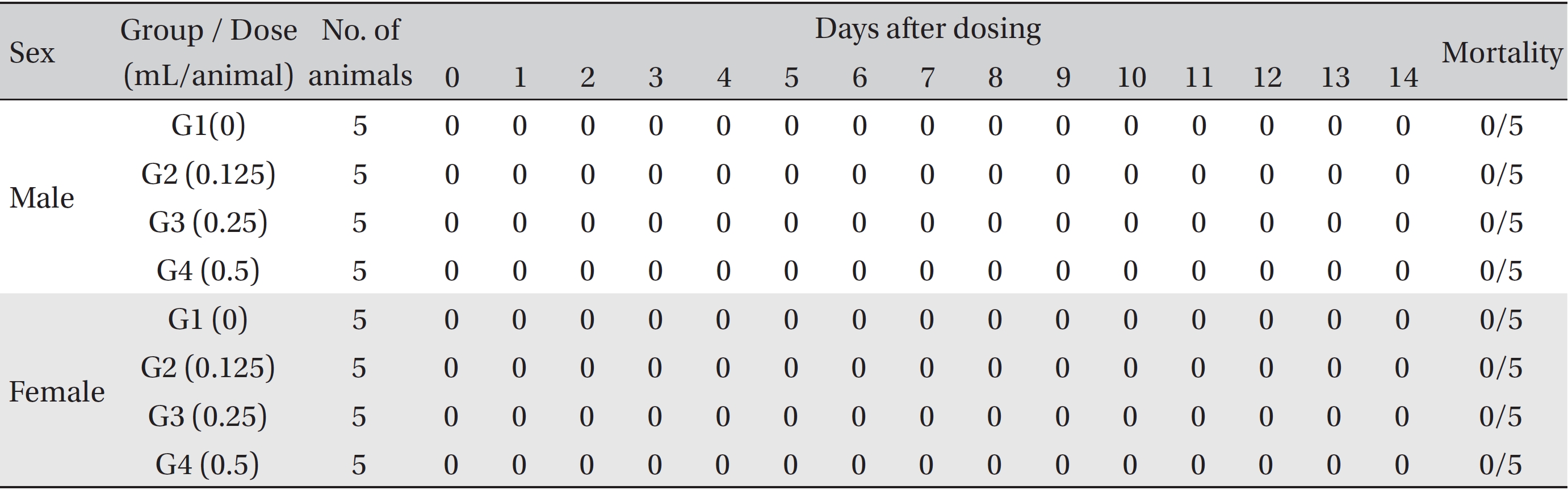
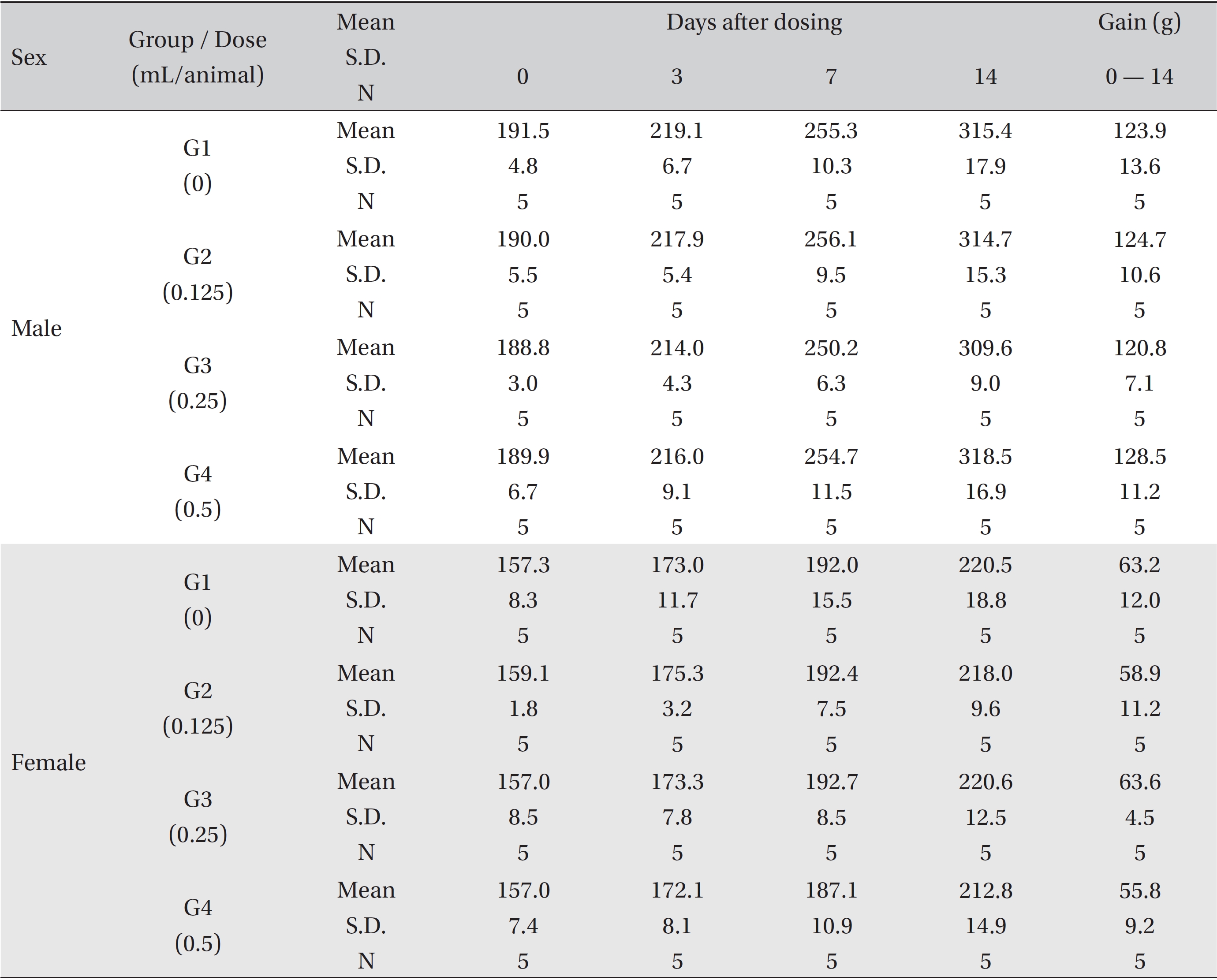
No mortalities were observed in any experimental group. No significant changes in weight, hematology, serum biochemistry, and histological observations that could be attributed to the intramuscular injection of BVP were observed in any experimental group.
Lethal dose of BVP administered via intramuscular injection in SD rats is over 0.5 mL/animal.
Twenty-four SD rats of each gender were obtained from Orientbio Inc. (Gyeong-gi, Korea) at 5 weeks of age and were used after a week of quarantine and acclimatization. The animals were housed in a room maintained at 21.1 — 24.1°C under a relative humidity of 40.7% — 64.5%. The room was illuminated with artificial lighting from 07:00 to 19:00 hours and had 10 — 15 air changes per hour. Three animals per cage were housed in suspended stainless-steel wire-mesh cages and were allowed sterilized tap water and commercial rodent chow (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C, Harlan Laboratories, Inc., U.S.A.). This study protocol was approved by the institutional Animal Care Committee of Biotoxtech Co. (Oh Chang, Korea).
The BVP (Lot No. KPI-2013-01) was manufactured in a pathogen-free facility at the Korean Pharmacopuncture Institute, Seoul, Korea by using BV purchased from Shandong, China. Then, the pharmacopuncture with a concentration of 0.1 mg BV/mL was filtered through 0.1-μm filter paper. Finally, the BVP was sterilized before being used for this experiment.
Twenty healthy male and 20 healthy female SD rats were selected from among the original 48 SD rats and were assigned to 1 of 4 groups with five male and five female SD rats per group: control (normal saline at 0.5 mL/animal), low-dosage (BVP at 0.125 mL/animal), medium-dosage (BVP at 0.25 mL/animal) and high-dosage (BVP at 0.5 mL/animal) groups. BVP or normal saline (Lot No. 12133, Choongwae Pharma Corp., Korea) was administered to the rats by intramuscular injection in the left thigh.
All animals were observed for clinical signs at 30 minutes, 1 hour and 2 hours immediately after the injection and once a day, starting the day after injection, for 14 days. The body weight of each rat was measured at the beginning of treatment and at 3 days, 7 days and 14 days after the injection.
On day 15 after treatment, the animals were fasted for 18 hours prior to necropsy and blood collection. Blood samples were drawn from the abdominal aorta under isoflurane anesthesia by using a syringe needle. Blood samples were collected into tubes containing ethylenediaminetetraacetic acid (EDTA) and were analyzed to determine the red blood cell count (RBC), hemoglobin concentration (Hb), hematocrits (Ht), mean corpuscular cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular cell hemoglobin concentration (MCHC), platelet count, white blood cell count (WBC), differential WBC count, reticulocyte (Reti) count, prothrombin time (PT) and active partial thromboplastin time (APTT) by using Hematology Systems (ADVIA 2120i, Siemens, Munich, Germany).
For the serum biochemistry analysis, blood samples were centrifuged at 3,000 rpm for 10 minutes and analyzed by using an auto-analyzer (7180, Hitachi, Tokyo, Japan). Serum biochemistry parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine, total bilirubin, total protein (TP), albumin, albumin/globulin ratio (A/G ratio), total cholesterol (T-Chol), triglycerides (TG), phosphate (P), glucose (Glu), calcium (Ca), chloride (Cl), sodium (Na) and potassium (K), were examined.
The tissues from the left thighs of the rats were routinely processed, embedded in paraffin and sectioned into 3- to 5-μm pieces. The sections were stained with hematoxylin and eosin (H&E) for microscopic examination. All tissues taken from all animals were examined microscopically.
Data on animal weights and on their blood chemistry and hematology were tested by using a statistical analysis system (SAS, version 9.3, SAS Institute, Inc., Cary, NC, U.S.A.). The variance in the numerical data was checked by using the Bartlett test. If the variance was homogeneous, the data were subjected to a one-way analysis of variance (ANOVA). If either of the tests showed a significant difference among the groups, the data were analyzed using the multiple comparison procedure of the Dunnett test. If not, they were analyzed using the Kruskal-Wallis non-parametric ANOVA test (
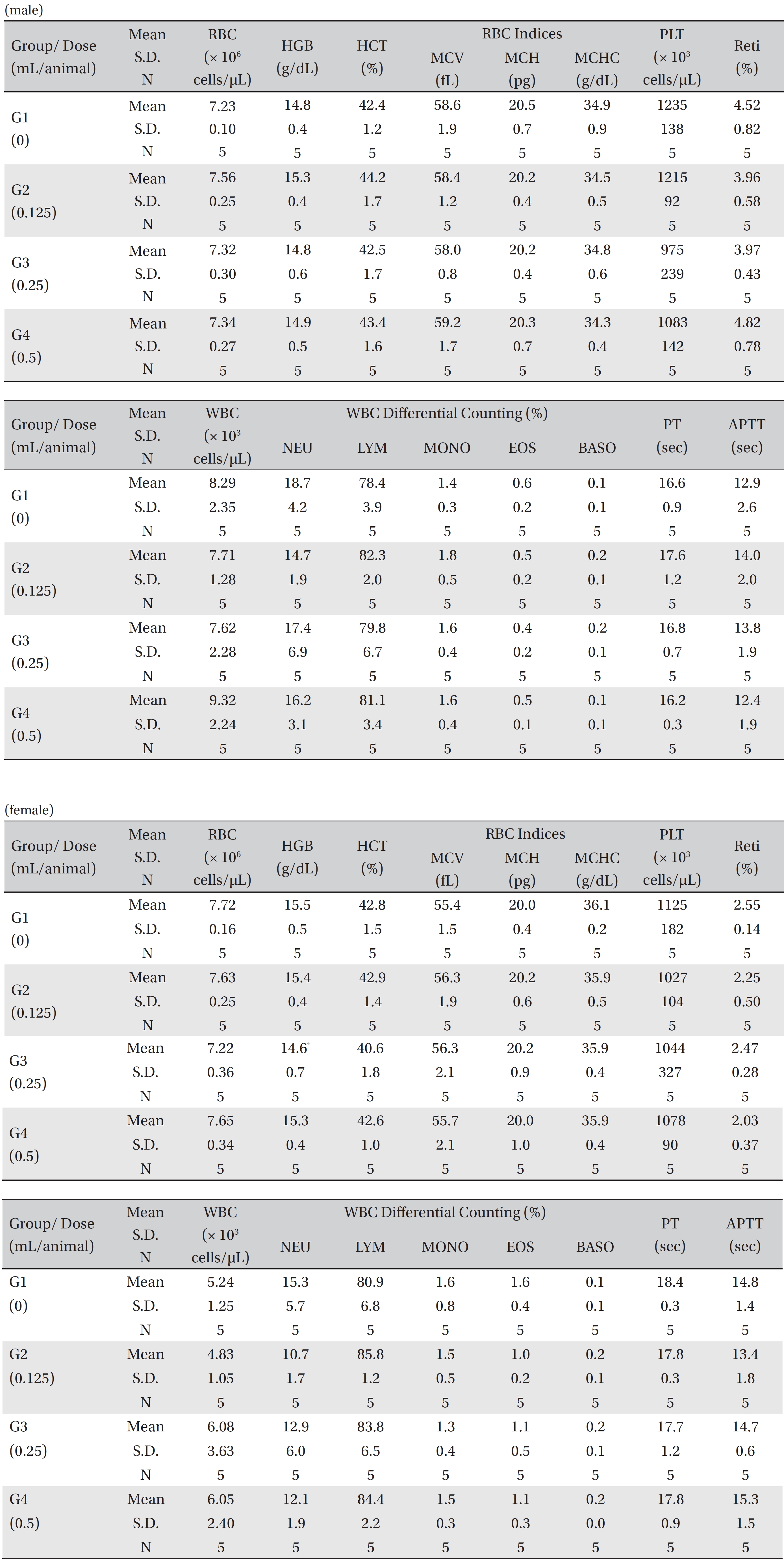
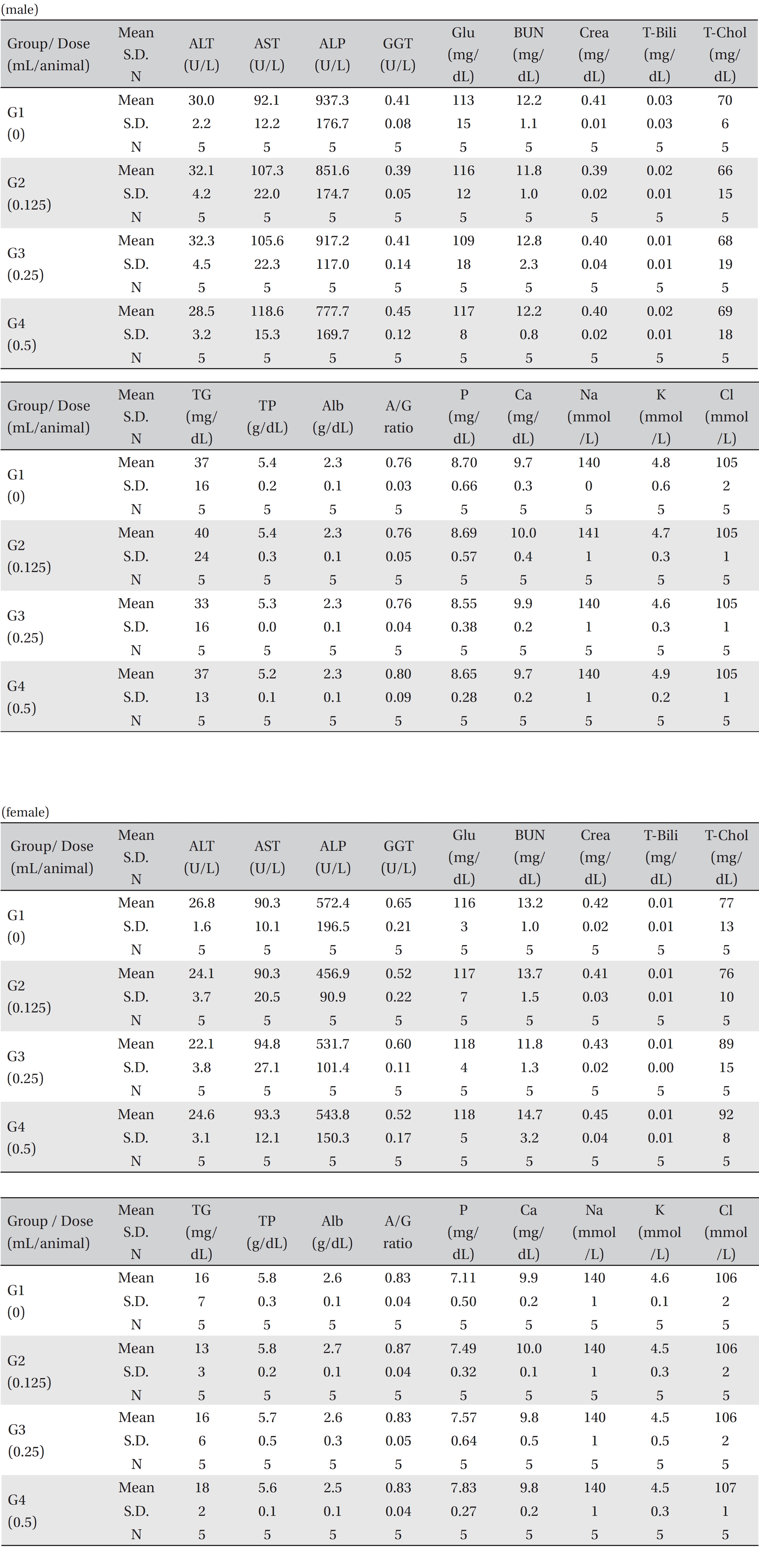
No treatment-related mortalities, clinical signs or weight changes occurred in either the control animals or the animals treated with any dose of BVP during the observation period (Tables 1,2). On the hematological examination (Table 3), one female in the medium-dosage group showed a significant change; however, the change was not dose-dependent; the change seemed to have occurred sporadically. The blood chemistry tests (Table 4) showed no significant changes.
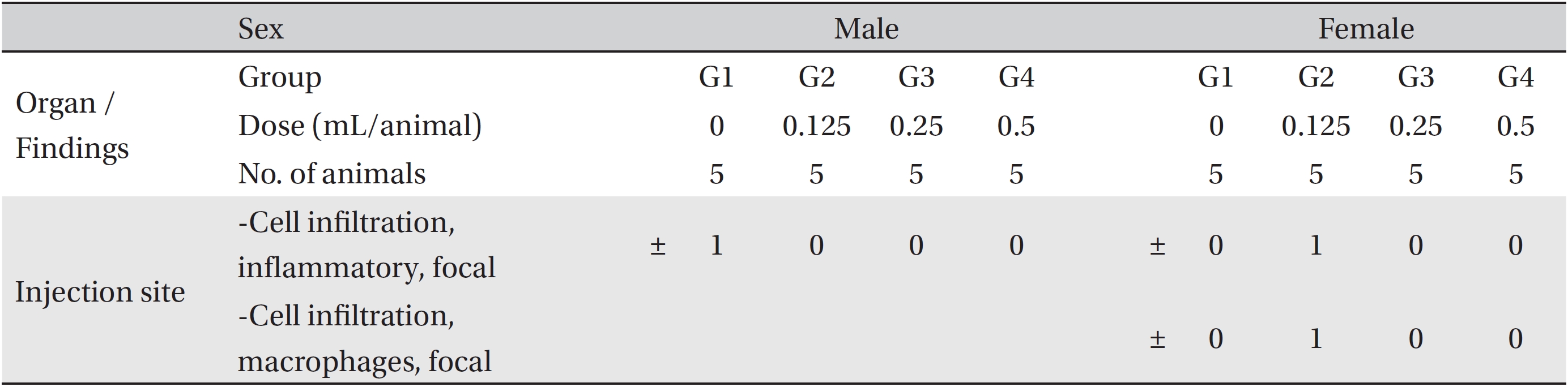
The necropsy examinations (Tables 5,6) showed no abnormalities. Moreover, on the histopathological examination, one male in the control group and one female in the low-dosage group showed abnormal changes, but those changes were not dose-dependent. Thus, they were deemed not be important toxicological changes.
BV has some toxic ingredients that can induce serious effects, including bradycardia, atrioventricular conduction block, ventricular tachycardia, ventricular fibrillation, and sudden death [10]. Bufadienolides, such as bufalin, cinobufagin and resibufogenin, which are major sources of BV, are known to increase vasoconstriction, vascular resistance and blood pressure probably by inhibiting Na, K-adenosine triphosphate (ATP) ase activity [11, 12], and these substances have recently been reported to have a strong surface anesthetic activity, cytotoxic effect and differentiation- apoptosis activity on murine leukemia human acute promyeocytic leukemia (HL-60) cells [13]. In addition, BV includes bufotenine, an indole alkaloid that produces effects such as aphrodisia and hallucination, and serotonin, which is involved in various psychiatric disorders such as depression, anxiety, chronic obsession syndrome and impulsivity [14-16]. Thus, appropriate dosage and careful use are very important for the safe use of BV [2].
For the above reasons, we conducted an intramuscular single-dose toxicity test of BVP in SD rats to determine an appropriate dosage for its safe use. The results showed no treatment-related abnormalities for any of the used doses of BVP. The dose used for the high-dosage group was 0.5 mL/animal, and no dangerous signs were observed. Thus, we may conclude that 0.5 mL/animal of BVP is a safe dose in both male and female SD rats.
This study showed that the lethal dose of BVP was over 0.5 mL/animal in both male and female SD rats.
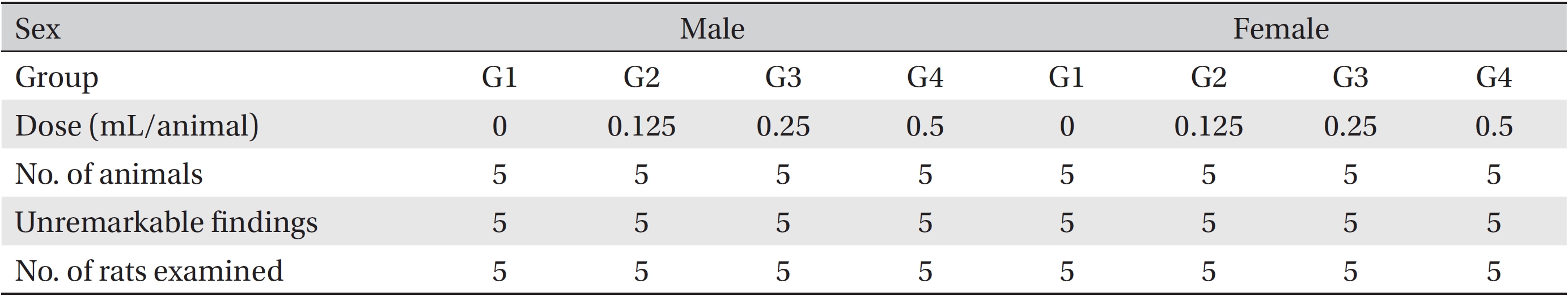
[Table. 1] Summary of mortalities
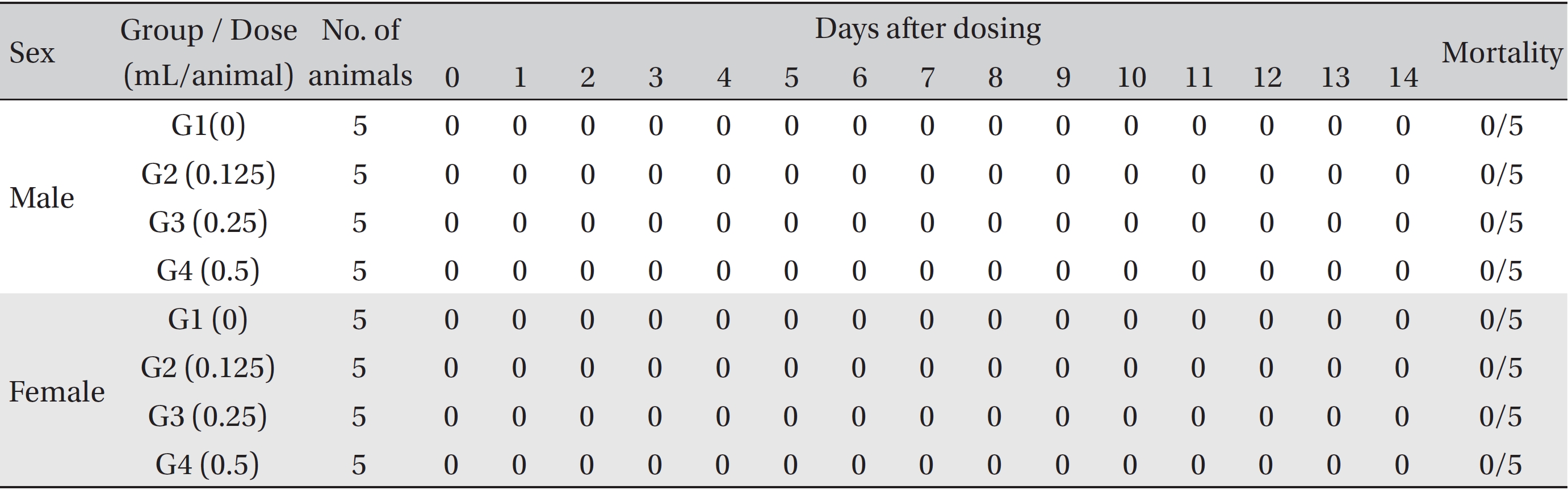
Summary of mortalities
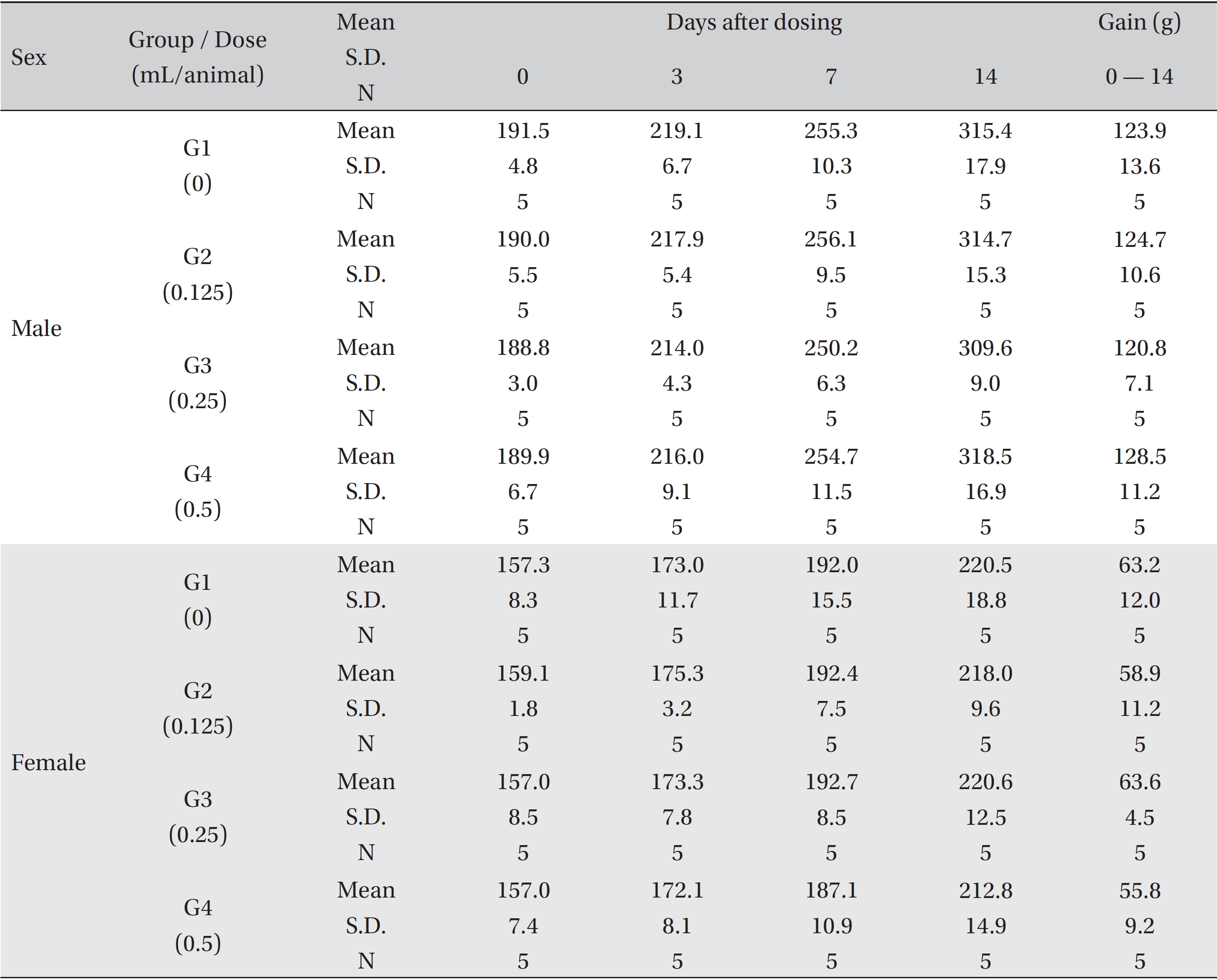
Mean body weights
[Table. 3] Mean hematology parameters
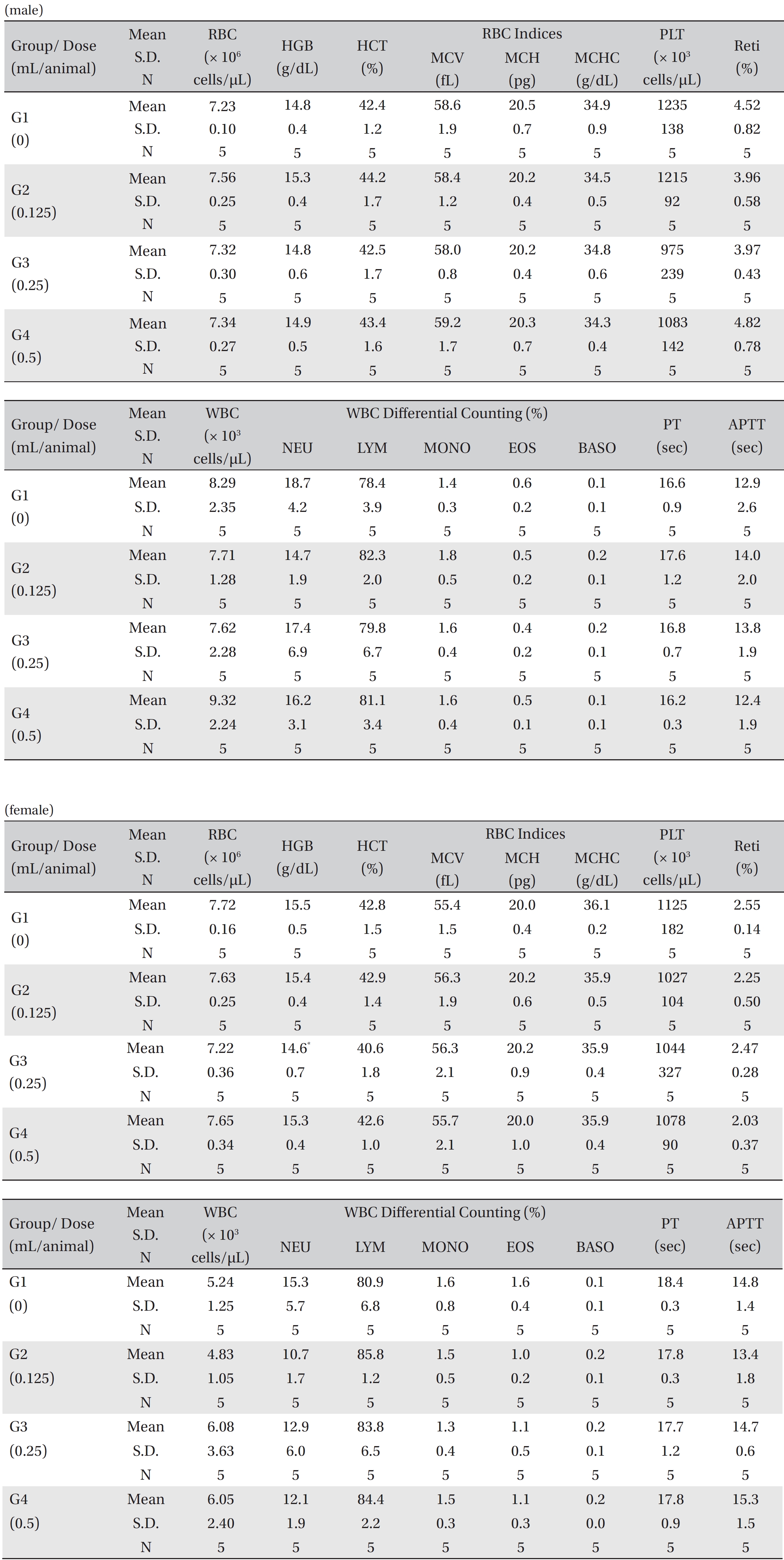
Mean hematology parameters
[Table. 4] Mean clinical chemistry
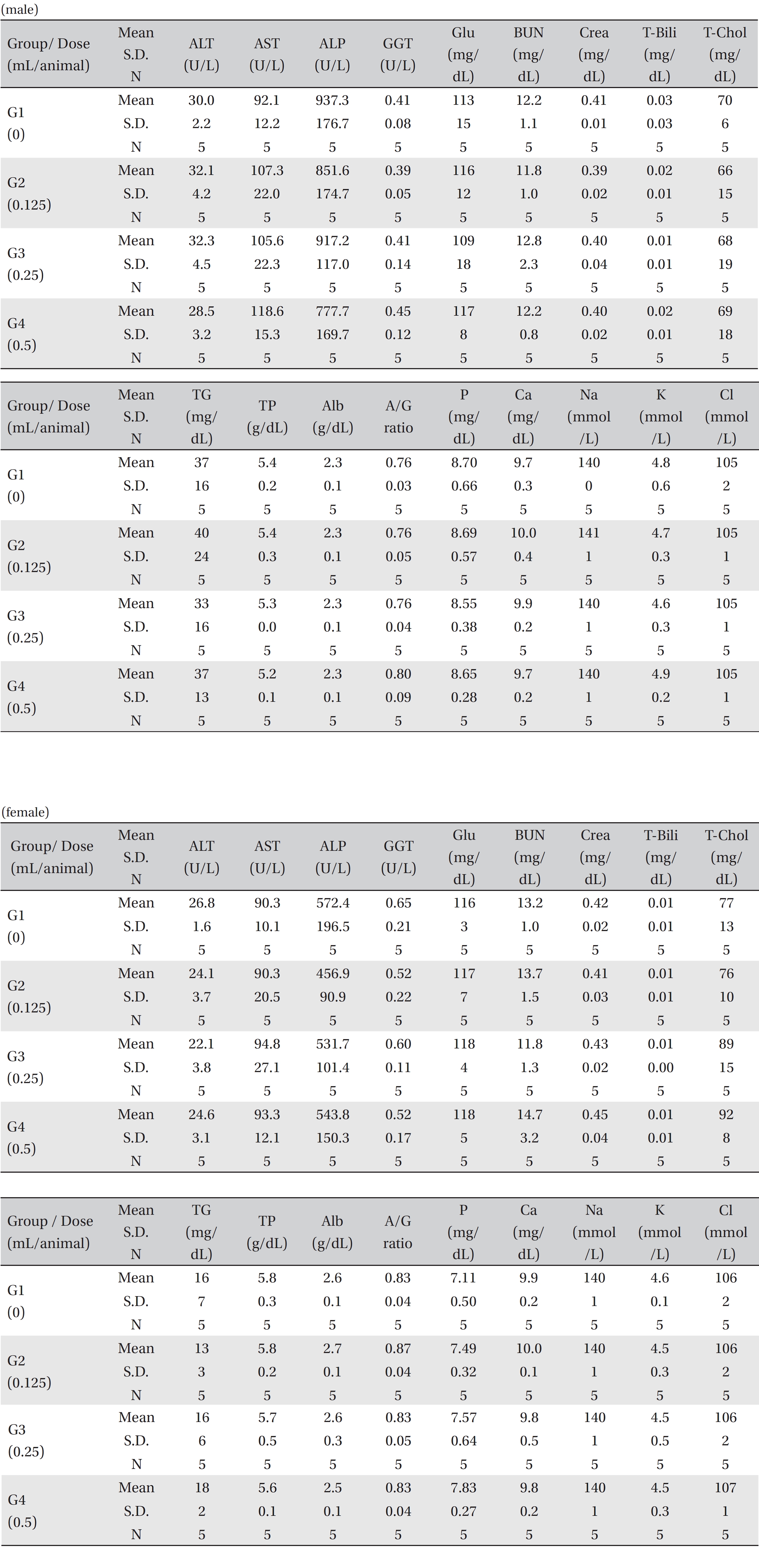
Mean clinical chemistry
[Table. 5] Summary of necropsy findings
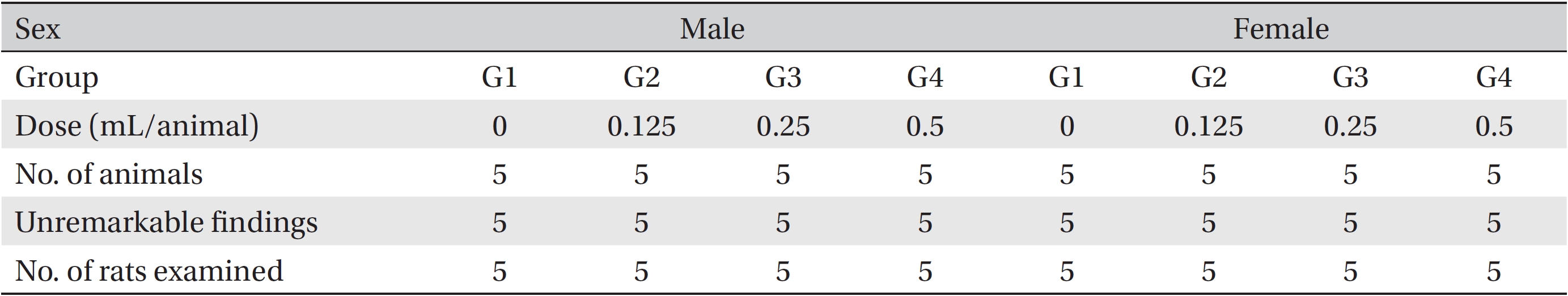
Summary of necropsy findings
[Table. 6] Summary of histopathological findings
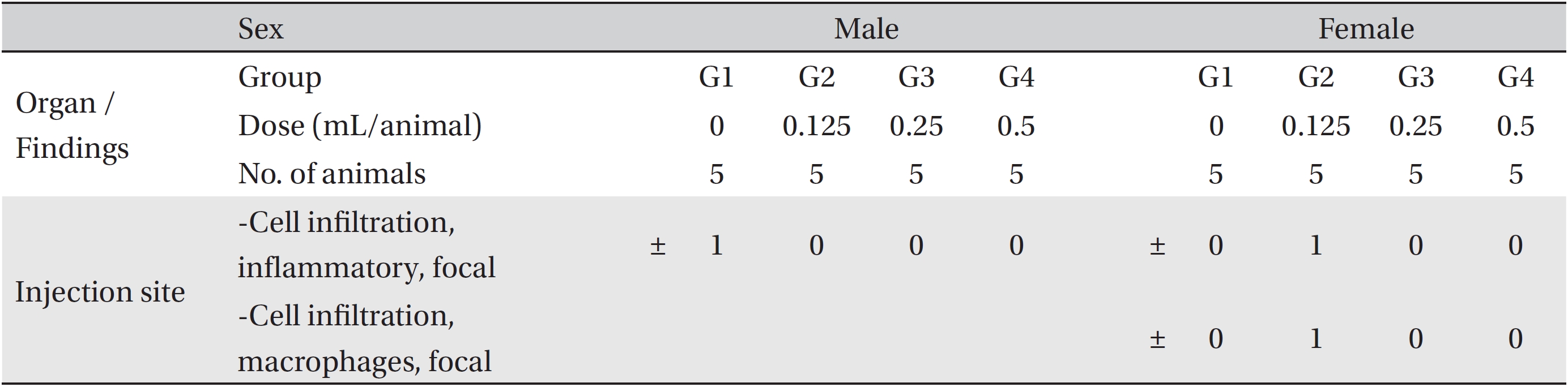
Summary of histopathological findings