The freshwater bryozoan, Pectinatella magnifica Leidy, commonly occurs in both lotic and lentic habitats. Most bryozoan species are found in relatively still waters in ponds, lakes, reservoirs, and slow-flowing streams, preferring shallower regions along the shores of lakes and ponds (Okamura and Hatton-Ellis 1995). They mainly live on a wide range of submersed substrates, including natural and artificial materials such as wood, rocks, glass, and plastics, and often in great numbers. Generally, bryozoan species do not grow effectively on soft substrates; thus, the availability of appropriate substrata for attachment is crucial (Tenney and Woolcott 1966, Wood 1989). In temperate regions, P. magnifica colonies grow rapidly during the warm months of the year, and can form dense mats on biological surfaces such as branches and aquatic macrophytes (Ryland 1970, Wood 1991). Their growth is largely restricted to relatively warm waters (15–28℃, mainly in summer), and although a wide pH range is tolerated, more alkaline waters tend to be favored (Hyman 1959, Wood 1989). Furthermore, the growth of P. magnifica is affected by dissolved oxygen (DO) levels, depth, and availability of food sources. Although bryozoan species have a wide range of tolerance, few P. magnifica occur in fairly eutrophic water bodies or waters with high concentrations of heavy metals (Henry et al. 1990, Wöss 1994). Therefore, P. magnifica colonies are generally difficult to find under highly polluted conditions.
Although the summer season is suitable for population growth of aquatic organisms such as P. magnifica, the climate of South Korea hinders the development and growth of aquatic organisms due to heavy rainfall during summer (Campbell 2002, Staley et al. 2007, Jeong et al. 2010). In particular, small animals such as rotifer and cladoceran are well known to be strongly influence by summer rainfall (Choi et al. 2011). However, there have been few studies on the effect of rainfall on P. magnifica colonies. We expected that P. magnifica colonies would be negatively affected by rainfall, consistent with other aquatic animals. Rainfall leads to increased water velocity and decreased residence time in river ecosystems (Jeong et al. 2007), which strongly influences the survival and population growth of aquatic animal communities. Freshwater bryozoans consume food items such as phytoplankton, rotifers, and protozoa, and they play a key role in regulating food web dynamics (Ryland 1970). In this regard, recognition of freshwater bryozoans has been central to limnological research.
The primary objective of our study was to investigate how the distribution pattern of P. magnifica is affected by summer rainfall. We investigated the contribution of substrate materials in supporting large colonies of P. magnifica during summer at three lotic freshwater ecosystems (Geum River, Miryang River, and Banbyeon Stream).
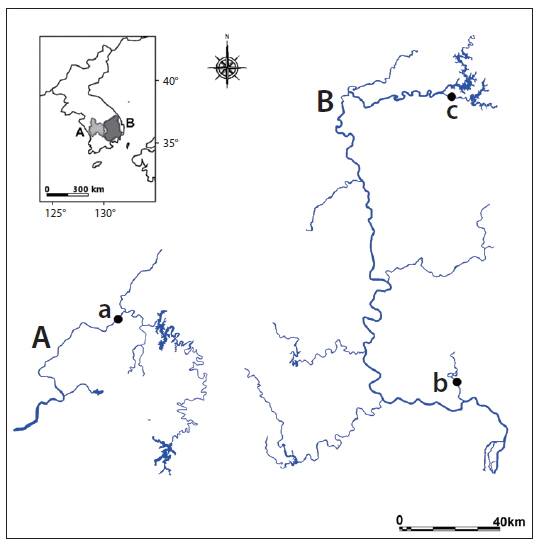
South Korea is located in East Asia, and has a temperate climate. Four distinct seasons lead to dynamic succession among biological communities inhabiting the country’s freshwater ecosystems. Rainfall in spring and early summer in 2014 was very low compared to previous years in the Geum River (mean rainfall in 2010 to 2014: 688.6 ± 120.3 mm, rainfall in 2014: 467.8 mm) and Nakdong River (mean: 557.2 ± 104.6 mm, 2014: 349.6 mm). Therefore, the growing season in 2014 was a more stable environment than previous growing seasons due to very low rainfall. In the present study, we monitored three sampling points with different environmental characteristics (Geum River, Miryang River, and Banbyeon Stream; Fig. 1 and Table 1). Sampling point 1 is located in the central-western part of South Korea, at the mid-region of the Geum River near Sejong Weir, and has slow water velocity and residence time. Sampling points 2 and 3 are located in the southeastern part of Korea, the mid-region of the Miryang River and the Banbyeon Stream. The Miryang River and the Banbyeon Stream are the main tributaries of the Nakdong River. The study sites located in the Geum and Miryang Rivers have abundant natural substrate material (e.g., stones and macrophytes), but the study site at the Banbyeon Stream is characterized by artificial bottom material (e.g., concrete). We used two selection criteria to estimate the influence of rainfall on P. magnifica colonies: (1) P. magnifica colonies should be attached to various substrate types, and (2) P. magnifica colonies should be abundant.
In this study, we investigated how the number of P. magnifica colonies on various substrate materials was affected during summer (July to August). Prior to collection of P. magnifica, we assessed the types of substrate material at each sampling point, and then counted the number of P. magnifica colonies on different types of substrate material (stones, macrophytes, branches, and artificial material, see Fig. 2) along the littoral area at each sampling point. The abundance and wet weight of P. magnifica attached to each substrate was expressed as the colony number per type of substrate material in a quadrat (1 × 1 m). This process was repeated ten times at each study site.
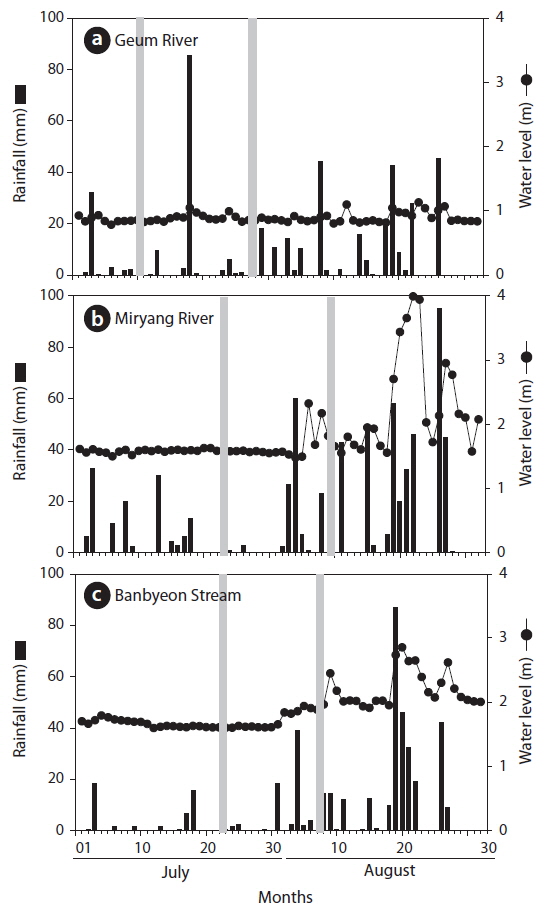
To understand the influence of rainfall on P. magnifica, we collected additional samples of P. magnifica colonies after rainfall at each sampling point. Fig. 3 shows the daily rainfall and sampling time at each sampling point. In addition, we collected P. magnifica colonies after 70–125 mm of rainfall. In the Miryang River and the Banbyeon Stream, water levels increased with rainfall, whereas in the Geum River there was only a minor effect of rainfall on water levels.
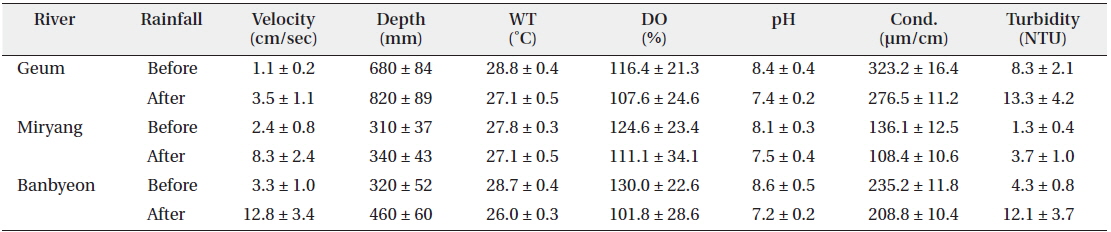
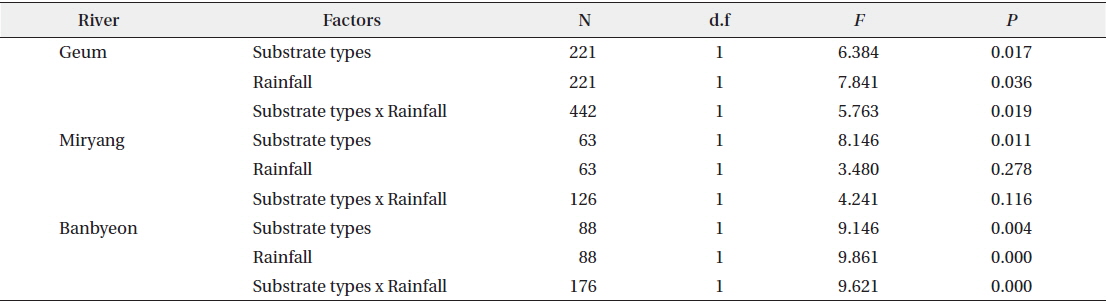
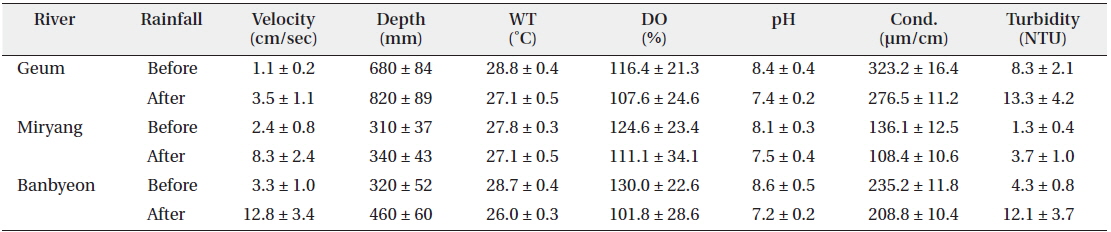
At a sampling point, we collected water samples using a 10 L water sampler to measure environmental variables. Water velocity was measured using a velocity instrument (Model 3631; Cosmo Riken Co., Yokogawa, Tokyo, Japan) along the riparian zone. Water depth was also measured three times along the littoral area. A DO meter (Model 58; YSI Inc., Yellow Springs, OH, USA) was used to measure the water temperature and DO, and conductivity and pH were measured using a conductivity meter (Fisher conductivity meter model 152; Fisher Instrument Co., Pittsburg, PA, USA) and pH meter (Orion Model 250A; Orion Research Inc., Boston, MA, USA), respectively. The collected water samples were transported to the laboratory to measure turbidity using a turbidimeter (Model 100B; HF Scientific Inc., Ft. Myers, FL, USA). We utilized a twoway ANOVA (α = 0.05) to compare the number of P. magnifica colonies in accordance with substrate type (stones, macrophytes, branches, and artificial material) and rainfall simultaneously using the statistical package SPSS for Windows (ver. 14).
In this study, environmental variables differed among study sites and were influenced by rainfall (Table 1). Velocity and water depth increased after rainfall at all study sites. In particular, water velocity before and after rainfall differed markedly in the Banbyeon Stream. In contrast, physicochemical variables such as water temperature, DO, pH, and conductivity decreased after rainfall at all study sites, whereas turbidity increased. Some studies have reported that complex combinations of exterior soil constituents and interior sediment disturbance could be involved in prolonged high turbidity during the rainy season (Kistemann et al. 2002). Moreover, Jeong et al. (2011) frequently observed prolonged high turbidity following rainfall during the summer in the Nakdong River. In general, aquatic animals (particularly zooplankton) sensitive to water flow are negatively influenced by physical disturbances such as rainfall, and thus, they mainly develop in spring and autumn (Choi et al. 2011). However, nutrient enrichment (nitrogen and phosphorus) was not related to bryozoan abundance (Hartikainen et al. 2009). In this study, we found that P. magnifica was strongly affected by increased water velocity following a rainfall event.
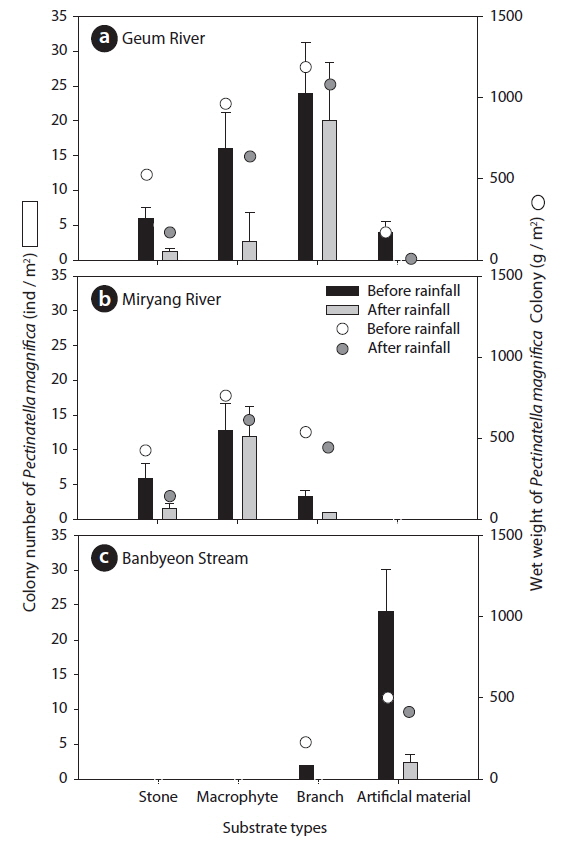
During the study period, we found a total of 372 colonies of P. magnifica: 221 colonies in the Geum River, 63 colonies in the Miryang River, and 88 colonies in the Banbyeon Stream. The number and wet weight of P. magnifica colonies differed in accordance with substrate type at each study site (Fig. 4). In the Geum River, P. magnifica colonies were more abundant on branches (average: 22 individuals/m2) than on other substrate types, followed by macrophytes (average: 8 individuals/m2). The wet weight of P. magnifica colonies was also higher on branches (average: 1,157 g/m2). We found a high abundance of branch material compared with other substrate types in the Geum River; thus, the branches support many colonies of P. magnifica. In addition, Hubschman (1970) suggested that wood material is an appropriate substrate for the attachment of P. magnifica colonies. In contrast, the Miryang River and Banbyeon Stream were characterized by a low abundance of branches, and thus, branch material supported relatively few colonies of P. magnifica. In all study sites, P. magnifica colonies were less frequently attached to stone material. Stones have a narrow substrate space, which reduces the attachment of P. magnifica colonies. In the Geum and Miryang Rivers, the amount of stone material was relatively small, and colonies of P. magnifica were sparsely developed on stone. There were also few colonies of P. magnifica found on artificial material (i.e., concrete) in the Geum and Miryang Rivers. Moreover, the colonies of P. magnifica on artificial material were small. However, the artificial material was more abundant in the Banbyeon Stream where it was able to support more number of colonies of P. magnifica of relatively larger sizes (>400 g/m2).
The influence of rainfall on P. magnifica colonies differed according to the type of substrate material (Fig. 4). In the Geum River, the numbers of P. magnifica colonies on stones and macrophytes markedly decreased after rainfall (stones: 6 and 2 individuals/m2; macrophytes: 16 and 3 individuals/m2, before and after rainfall, respectively), whereas there was no significant difference in the number of P. magnifica colonies on branches before (24 individuals/m2) and after (20 individuals/m2) rainfall. The wet weight of P. magnifica colonies had a similar trend as colony number. We found that branches can support more P. magnifica colonies against rainfall occurrence in summer. In contrast, in the Miryang River there were few colonies on branches after rainfall (3 and 1 individuals/m2, before and after rainfall, respectively). In the Miryang River, most of the branches were small, and P. magnifica colonized very few branches. However, macrophytes in the Miryang River supported similar numbers of P. magnifica colonies before (13 individuals/m2) and after (12 individuals/m2) rainfall. The wet weights of P. magnifica colonies on macrophytes and branches were no different in relation to rainfall occurrence. The Miryang River was dominated by macrophyte species with relatively complex structures, such as submerged and free-floating plants (e.g., Trapa japonica, Ceratophyllum demersum, and Vallisneria natans); thus, we propose that these plants could support a high abundance of epiphytic animals such as P. magnifica. Similarly, Choi et al. (2014a) suggested that epiphytic microinvertebrates were more abundant on submerged and free-floating plants than on other plant types. In particular, submerged plants markedly increase the physical complexity of aquatic environments, and provide a suitable habitat for colonization by aquatic animals (Manatunge et al. 2000, Choi et al. 2014b). Furthermore, Choi et al. (2012) suggested that macrophyte cover contributed to sustaining the number of aquatic animals following summer rainfall. However, emergent plant species (Phragmites communis and Typha orientalis), which have a relatively simple structure (Cazzanelli et al. 2008), dominated the Geum River; thus, we considered that emergent plants supported only a few colonies of P. magnifica.
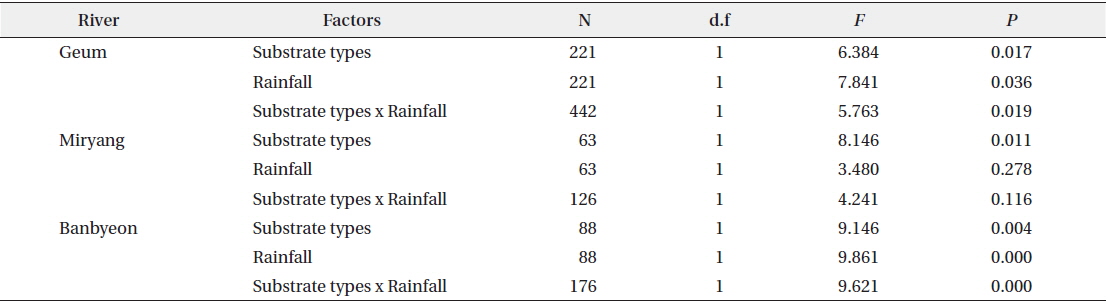
Two-way ANOVA revealed that colonies of P. magnifica were affected by both substrate type and rainfall (Table 2). In the Geum River and Banbyeon Stream, colonies of P. magnifica were significantly affected by both substrate type and rainfall, and there was a significant interaction between these two factors. This implies that substrate type determined the sustainability of P. magnifica colonies at varying levels of rainfall, resulting in high numbers of colonies on branches and macrophytes, and less number of colonies on stones and artificial materials.
On the basis of these results, we propose that the type of substrate material used is an important factor in determining P. magnifica distribution. Moreover, the influence of rainfall on P. magnifica colonies differed depending on the abundance and form of the substrate material. In the case of P. magnifica inhibition in river ecosystems, the selection of substrate type was more important for resistance to physical disturbances such as rainfall. Currently, Koreans have a negative impression of P. magnifica because of its frequent occurrence in river ecosystems. In spring (April to May) 2014, South Korea had low rainfall and high water temperature and pH; thus, we propose that such stable environments largely contributed to the growth and development of P. magnifica. Therefore, efficient countermeasures are needed to manage colonies of P. magnifica. In general, freshwater bryozoans commonly occur in lotic ecosystems such as lakes and reservoirs (Okamura and Hatton-Ellis 1995). On the basis of this information, we suspect that slow water velocity due to the presence of a weir prolonged residence time, and that this acted as a trigger for the proliferation of river bryozoans. We also found that colonies of P. magnifica were negatively affected by rapid water velocity and rainfall. Therefore, it is recommended that a water flow regime be maintained at near-natural levels to efficiently control P. magnifica colonies as well as their statoblasts and larvae.