The martens (genus Martes) are medium-sized animals belonging to Mustelidae, Carnivora; in total, 7 species are known worldwide. The martens are widespread in tropical, temperate, and boreal forest zones of the Old and New World. Martens are listed in the IUCN Red List (2014) as ‘Least Concern’ or ‘Vulnerable’ species (Buskirk 1994).
Yellow-throated marten, Martes flavigula Boddaert 1785, is brownish-yellow with golden-orange dorsum of the head, whereas forefeet, rear legs and the tail are dark brown or black. Body and tail lengths are 400–600 mm and 380–430 mm, respectively. The adult weight is approximately 3.4 kg and the life span is approximately 14 years. Yellow-throated martens mate in August and give birth in April (Lekagul and McNeely 1977, Nowak 1999). M. flavigula moves in groups of 2 or occasionally 3 (Fig. 1a), unlike the other six Martes species, which are solitary (Grassman et al. 2005, Choi et al. 2009). M. flavigula inhabits sub-tropical and temperate forests of the Far East and Southeast Asia, southern China, the Himalayas, and India (Prater 1971, Harrison et al. 2004, Proulx et al. 2004).
M. flavigula is found across the whole Korean peninsula with an estimated population of 2000–3000 individuals based on presumed 50–60 individuals in Jirisan National Park (Choi et al. 2011). Despite its ‘Least Concern’ or ‘Vulnerable’ status in the IUCN Red List (2014), M. flavigula is considered to be an endangered species (Class II) by the Ministry of Environment of South Korea. M. flavigula is a top-level predator that plays the role of an umbrella species for conservation of the forest ecosystem and biodiversity because there are no large predators in the ecosystems of South Korea (Choi et al. 2009, 2010).
Although martens mainly feed on rodents and fruits (De Marinis and Masseti 1995, Zhou et al. 2010), their diet varies with the location and seasons (Zhou et al. 2010, 2011). Martens, including M. flavigula, are omnivorous and feed on fruits, rodents, birds, amphibians, reptiles, and insects (Lekagul and McNeely 1977, Clevenger 1994, Harrison et al. 2004, Parr and Duckworth 2007, Zhou et al. 2010, 2011). Like other martens, M. flavigula is considered to be an important seed disperser (Zhou et al. 2008). Choi et al (2009, 2012) reported that groups of M. flavigula forage on relatively large animals such as roe deer, young wild boar, and water deer. The diet of M. flavigula varies with prey resources in the environment. This dietary plasticity indicates adaptability of this marten species to new habitats (Zhou et al. 2008).
The composition of the martens’ diet has been extensively studied, but little is known about its insect component. This study characterized the insect diet of M. flavigula adapted to Korean ecosystems by identifying the species of seasonal insects in the feces of M. flavigula.
This study was conducted in Jirisan National Park in South Korea, which contains 471,758 km2 of a typical temperate forest (Fig. 2). The feces of M. flavigula were collected along the Mt. Jirisan ridge. M. flavigula marked their range by placing their feces on small rocks and fallen trees (Fig. 1b; Choi et al. 2009). The dates and coordinates of collection sites were recorded, and the feces were placed in plastic bags and stored at −80℃. Sample collection was conducted monthly from January 2009 to November 2011.
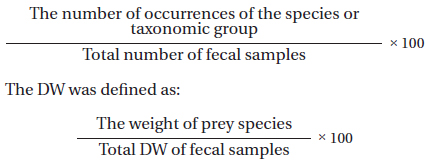
Analyses of fecal samples were performed according to the method of Jedrejewska and Jedrzejewski (1998). The contents of frozen feces were extracted by incubating them in water for 3 days; the samples were then filtered through a mesh sieve (5 mm). The matter that remained in the sieve was dried in an oven for 5 h at 50℃ and insect taxa were identified under a microscope. Social wasps were identified according to Yamane et al. (1980), Kim et al (2006), and Archer (2012). The insect diet composition was expressed in two ways: as the frequency of occurrence (FO) and dry weight (DW) of biomass. The FO was calculated according to Balestrieri et al. (2011) and Zhou et al. (2011) as:
Two-way PERMANOVA (permutation multivariate analysis of variance) was used to test the independent and interactive effects between the seasons and frequency of insects of each taxonomic group. The non-parametric PERMANOVA test calculates a pseudo-F (based on permutations), which is comparable to the F statistic from ANOVA and is not affected by non-normal distributions of data. The Euclidean distance measure was used. Multivariate analyses were performed in PAST (Hammer et al. 2001) using 9999 permutations.
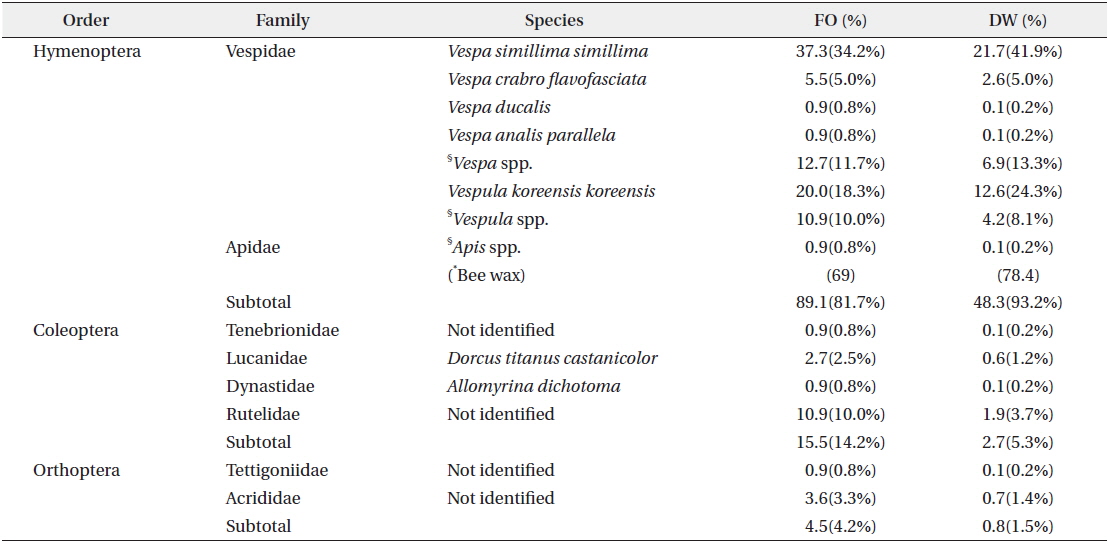
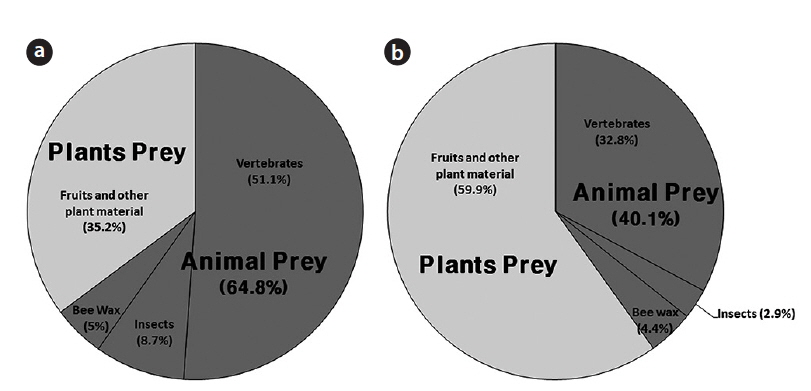
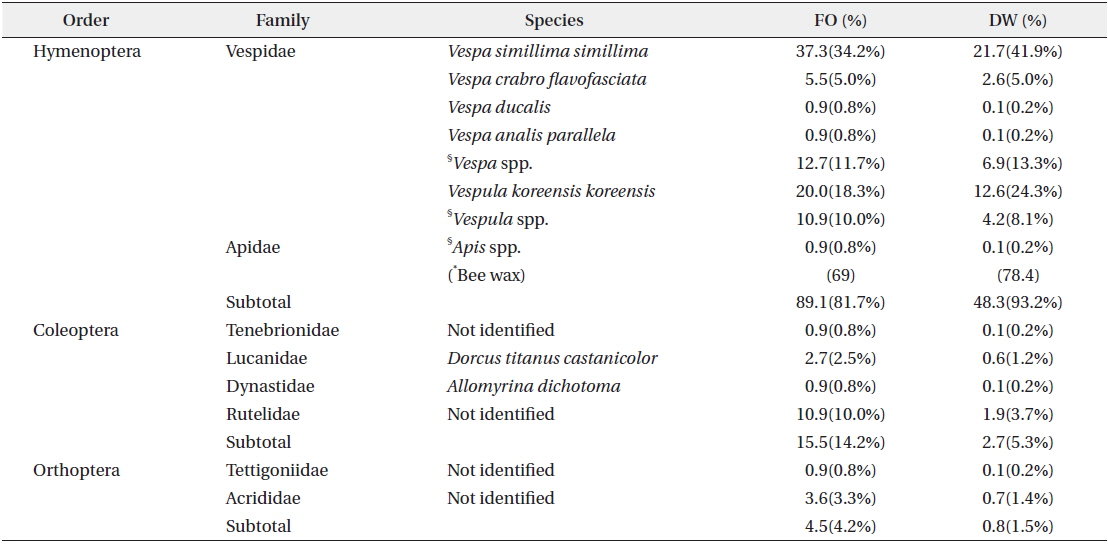
In total, 952 fecal samples were collected during the study period. Among the total 1379 species or taxonomic groups identified, we detected both animals (dietary composition, DC, 64.8%; DW, 40.1%) and plants (DC, 35.2%; DW 59.9%). Among the remains of animal origin, we detected vertebrates (DC, 51.1%; DW, 32.8%), insects (DC, 8.7%; DW, 2.9%), and bee wax (DC, 5.0%; DW, 4.4%) (Fig. 3). A total of 12 insect species belonging to 8 families in 3 orders were identified (Table 1). The most frequent were Hymenoptera (FO, 89.1), especially Vespa simillima simillima (FO, 37.3) (Fig. 4a) and Vespula koreensis koreensis (FO, 20.0) (Fig. 4b), followed by Coleoptera (FO, 15.5) (Fig. 4e), such as Dorcus titanus and Allomyrina dichotoma, and Orthoptera (FO, 4.5) (Fig. 4d). Although we did not consider bee wax as a component of the insect diet, its FO was 69; honeybees were not found (Table 1 and Fig. 4c).
Total DW of the insect diet in collected fecal samples was 51.8 g. The Vespidae constituted 48.2 g (93.0%). Coleoptera and Orthoptera accounted for 2.7 g (5.3%) and 0.8 g (1.5%), respectively (Table 1).
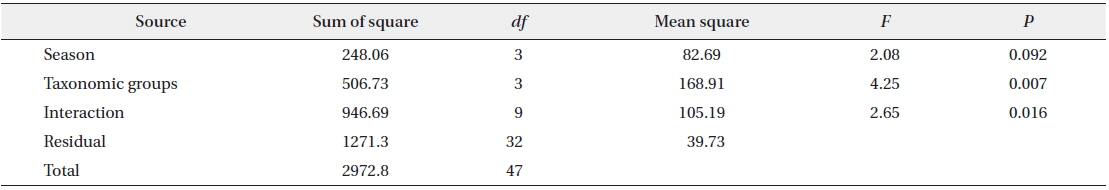
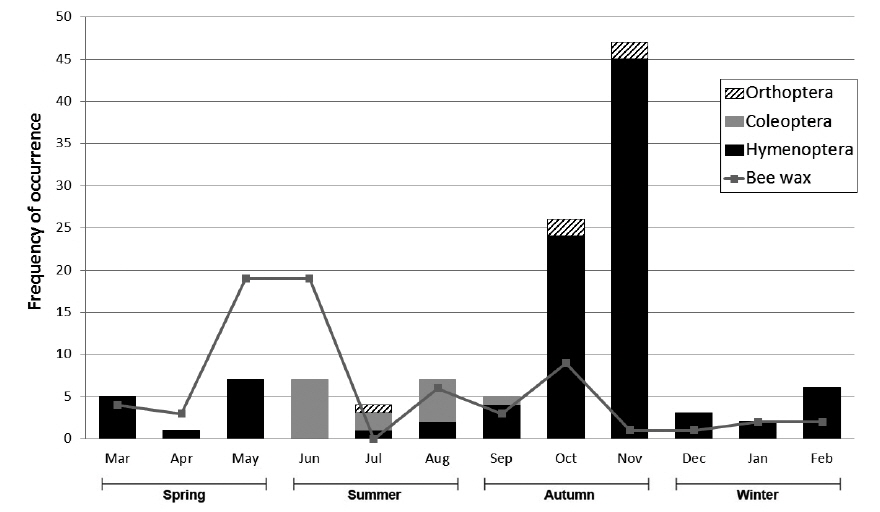
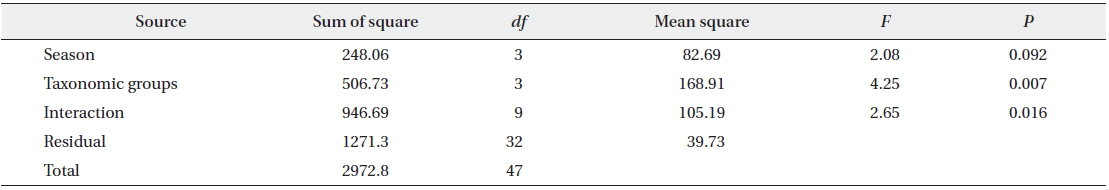
M. flavigula fed only on Vespidae in winter and spring, and also consumed Coleoptera and Orthoptera in summer and autumn. In autumn, Vespidae made up most of its insect diet, as their numbers rapidly increase in late summer and autumn. Bee wax appeared in the diet of M. flavigula in all four seasons and the highest relative percentage was found from spring to early summer (Fig. 5). However, the frequency of insect occurrence did not differ significantly among seasons (PERMANOVA, F = 2.08, P = 0.092), but differed significantly among taxonomic groups (F = 4.25, P = 0.007). The interaction between seasons and taxonomic groups was significant (F = 2.65, P = 0.016) (Table 2).
In this study, we characterized the insect diet of M. flavigula in different seasons. Even though insects were not the main diet of martens, they were consumed throughout the year and included Hymenoptera, Coleoptera, Orthoptera, Homoptera, and Hemiptera (Zhou et al. 2011). The ratio of insects to other components of martens’ diet was considerable (about 10-30%) (Zhou et al. 2010). The two most frequently detected taxa were Hymenoptera and Coleoptera, which were in abundance from spring to autumn (Marchesi and Mermod 1989, Clevenger 1993, 1994, De Marinis and Masseti 1995, Zhou et al. 2010, 2011, Balestrieri et al. 2011,). Besides insects, M. flavigula fed a beehive because of its fondness for honey (Lekagul and McNeely 1977, Payne et al. 1985). These results are in accordance with our expectation that insects are eaten by martens because they are easy to forage for and are available all year round. According to Zhou et al. (2011), martens feed on fruits despite their relatively low protein content because fruits can be easily procured relative to time and effort invested to forage for mammals. Similarly, martens meet their protein requirements by consuming insects during the periods of food shortages.
We found that insects occupied a smaller fraction in M. flavigula feces (<10%) than vertebrates and fruits. Hymenoptera are generally dominated by Apoidea or wide bees (Roberts 1977, Zhou et al. 2011). However, we found that M. flavigula primarily preys on social wasps, including hornets and yellow jackets, together with their nest materials. This is probably because social insects can supply sufficient food for M. flavigula, which would be impossible with solitary insects. There are hundreds to thousands of larvae, pupae and adults in the nests of social wasps, which are high in protein (Matsuura and Yamane 1990). As we expected, V. simillima simillima was the most important food resource for M. flavigula since their nests are attached to tree branches where the nests are usually found by martens. Vespa crabro, V. ducalis, V. analis, and Vespula species usually build their nests in tree crevices, tree branches or bushes, or underground (at a depth of 10-20 cm) (Matsuura and Yamane 1990), where they are also accessible to M. flavigula. However, most Vespidae species have a strong venomous sting, which is very dangerous against animals. M. flavigula has a strategy to minimize the exposure to Vespidae stings. Fig. 5 shows that the numbers of Coleoptera, Orthoptera, and Hymenoptera captured by M. flavigula were low in summer and early autumn (September) when sufficient prey is available. However, the amounts of Hymenoptera captured increased dramatically in October and peaked in November. The life cycle of the Vespidae begins with a queen building an embryo nest in early spring (Matsuura and Yamane 1990). The first workers continue nest building in June, and the nest then grows rapidly. The nest size is maximal in August and September, when large numbers of aggressive workers are sufficient to protect it from predators. New reproductives are produced after September; however, at that time males do not have a venomous sting and queens are less aggressive than workers. Therefore, it seems that M. flavigula frequently attacks the nests of social wasps in late autumn when the amount of larvae and adults increases and their aggressive behavior is reduced. Because prey is insufficient in winter and early spring, M. flavigula forages easily on overwintered new queens found in tree crevices or underground, embryo nests, and beehives. Unlike Vespidae, however, the remains of honeybees were not found in the feces; therefore, M. flavigula presumably eats honey but not honeybees. Although this study could not fully address the mechanism of M. flavigula foraging, it should be studied in the future.