Freshwater wetlands provide a stable habitat due to their low water velocity and shallow depth (Denny 1994, Findlay and Bourdages 2000, Eppink et al. 2004). In particular, a diverse range of macrophytes can easily develop in wetlands, and these macrophytes play an important role in structuring habitats in aquatic ecosystems (Agostinho et al. 2007, Meerhoff et al. 2007). Some studies have suggested that vegetated beds with high structural heterogeneity provide refuge for small animals against predators and suitable spawning and foraging substrates, mediating trophic interactions between diverse organisms (Vieira et al. 2007, Thomaz et al. 2008).
Macrophyte habitat structure is determined by the number of individuals, morphology, and arrangement of stems, branches, and leaves (Lillie and Budd 1992), and this architecture may influence the species composition and abundance of diverse organisms residing in the beds. Colonization of macrophyte species with a diverse morphology constructs more complex habitat structure, thus supporting a higher diversity of aquatic animals. Furthermore, the morphology of macrophytes has a significant bearing upon aquatic animals as food source provision, such as detritus trapping (Rooke 1984) and support of periphytic algal growth (Cattaneo et al. 1998).
Among the various life forms of macrophytes, submerged macrophytes were particularly increased the physical complexity of aquatic environments. The majority of previous studies, which aimed to understand the trophic interactions between fishes and invertebrates in vegetated habitats, only considered the role of submerged macrophytes (Jeppesen et al. 1998). Submerged macrophytes are capable of providing a suitable habitat for aquatic animals through an increase in refuge space in the water. Recently, the importance of surface-dwelling macrophytes (e.g., free-floating- and floating-leaved macrophytes) with respect to habitat structure has also been emphasized in some freshwater ecosystems, such as subtropical lagoons (Meerhoff et al. 2003). Moss et al. (1998) found a high biomass of plant-attached animal species in floating-leaved macrophytes. On the basis of this evidence, we expect that the composition of animal species residing in habitats created by macrophytes may be influenced by different types of macrophyte structure.
In South Korea, the majority of wetlands present a mixed colonization of different types of macrophytes (i.e., contemporaneous occurrence of submerged and surfacedwelling macrophytes). Unfortunately, previous studies have only focused on a single type of macrophyte life form, and the influence of macrophyte mixtures has not been seriously scrutinized. We hypothesize that mixed plant zones may form more complex habitats, resulting in greater faunal diversity and more complex food web interactions.
The main purpose of the present study was to explore the abundance and interactions among aquatic organisms living in different types of macrophyte beds (i.e., reed zones and mixed plant zones). We expected different species composition of aquatic organisms between reed and mixed plant zone. Moreover, stable isotope analysis was conducted to define the different interaction of aquatic organisms in the different macrophyte zones. We compared the composition and the food web of aquatic organisms between the reed- and mixed plant zone.
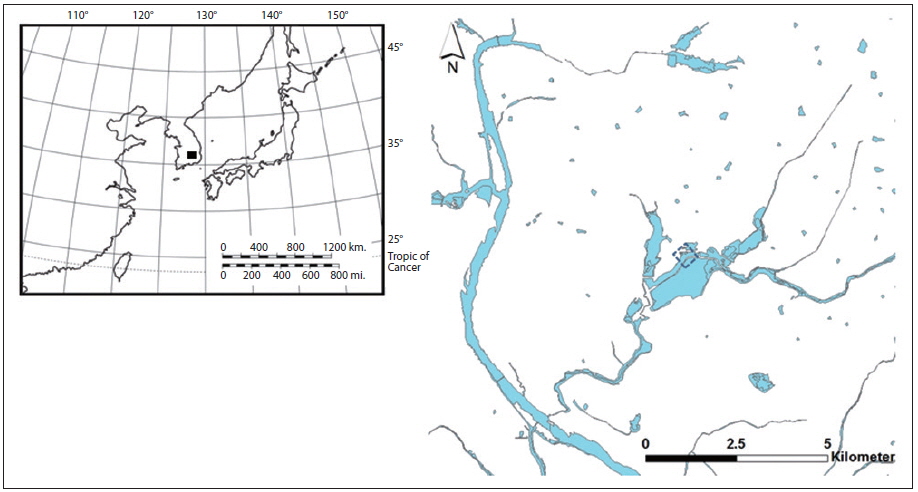
South Korea is located in the East Asian region, and experiences a temperate climate. Four distinct seasons lead to dynamic succession among biological communities in the freshwater ecosystems of Korea. The Upo Wetland, monitored in this study, is located in southeast Korea (Fig. 1), in the mid- to low-catchment of a large river (the Nakdong River). The surface water area covers 2.7 km2 and the average depth is as shallow as 0.8 to 1.4 m. Our sampling area was located in the north part of the Upo Wetland where a distinct division of the plant assemblage could be observed. The shallowest area of the wetland, near the shoreline, is mostly dominated by
A quarterly sampling program was implemented (spring, May; summer, August; autumn, November; winter, February) in the Upo Wetland by means of quadrat sampling (size, 1 m × 1 m). A total of 6 quadrats were maintained throughout the study period and these were evenly divided into two main groups, the reed zone and the mixed-plant zone (i.e., 3 quadrats in the reed zone and 3 quadrats in the mixed plant zone). At each sampling point, the geographical location of the sampling point within the quadrat was recorded to avoid accidental resampling of a previously sampled point. Also, we selected three sampling point where species composition of macrophyte and depth were similar. Within each quadrat, aquatic organisms (zooplankton, macro-invertebrates, and fish) were collected along with suspended and epiphytic particulate organic matter (SPOM and EPOM, respectively). SPOM samples were obtained by collecting a 2-L water sample from each quadrat, and they were filtered by using GF/F glass fiber filters (pore size, 0.45 μm; pre-combusted at 500℃ for 2 h). EPOM samples were obtained by scraping the surface of the macrophyte leaves and stems using a brush. The scraped plants were dried at 60℃ for 2 days to measure gram dry weight (gdw). Units of SPOM were micrograms per liter (μg L-1), and EPOM concentration was expressed as micrograms per gram dry weight (gdw) of plant (μg gdw-1). The POM samples were divided into two sub-samples to measure chlorophyll a concentration and stable isotope signals. Chlorophyll a concentration was measured based on a protocol in Wetzel and Likens (2000). The POM samples for stable isotope analysis were treated with hydrochloric acid (1 mol/L HCl) for 48 h to remove inorganic carbon and rinsed with distilled water to remove the acid.
For zooplankton sample preparation, 5-L water samples were collected using a 5-L column sampler in each quadrat, and the samples were filtered through a plankton net (32-μm mesh). Zooplankton samples for identification and enumeration were fixed with formaldehyde (final concentration, approximately 4%; Haney and Hall 1973), and the classification key provided by Mizuno and Takahashi (1991) was followed. The samples for stable isotope analysis were sorted manually using a fine pipette, and the individual species were separated from other organic matter. Macro-invertebrate collection was also conducted for approximately 30 to 40 min using a stainless steel sampler (200 mm diameter, 600-μm mesh size) in each quadrat. All macro-invertebrates within each quadrat were collected. The collected macro-invertebrates and organic material, including plant debris, were preserved in 4% formaldehyde solution. In the laboratory, samples were sorted into taxonomic groups according to Merritt and Cummins (1996) by the unaided eye. However, the macro-invertebrate samples for stable isotope analysis were stored as order or family level without formaldehyde preservation. After collection of zooplankton and macroinvertebrates, fish were collected using a cast net (20 times within 30 min) and a scoop net (approximately 20 min) around the quadrat. Individual fish were immediately identified after catching, and then 3 to 4 individuals per species were randomly selected to obtain muscular tissue from the fish body for stable isotope analysis. The muscular tissue samples were divided into two sub-samples for carbon and nitrogen isotope analysis. Because lipids present in muscular tissue can affect the reliability of the carbon isotope signature, lipids were extracted from all samples in the first sub-sample using a solution of methanol- chloroform-triple-distilled water (2:1:0.8, v/v/v) for 24 h. The lipid extraction affects nitrogen isotope signature; thus, the other sub-samples were utilized in nitrogen signature detection, without lipid extraction process. The lipid extraction process was conducted on not only fish but also zooplankton and micro-invertebrate.
All pre-treatment samples were freeze-dried and homogenized in a mortar using a pestle, and then the powdered samples were frozen at -70 °C until analysis. Carbon and nitrogen isotope ratios were determined using continuous-flow isotope mass spectrometry. Dried samples (ca. 0.5 to 1.0 mg) were combusted in an elemental analyzer (EuroVector, Milan, Italy) and the resultant gas (CO2 and N2) was introduced to a Micromass IsoPrime isotope ratio mass spectrometer (CF-IRMS, ABCA; PDZ Europa, Crewe, UK) in a continuous flow using a helium carrier. Data were expressed as the relative concentration (‰) difference between sample and conventional standards of Pee Dee Belemnite carbonate (PDB) for carbon and air N2 for nitrogen, according to the following equation:
δ X (‰) = [(
where X is 13C or 15N and
A one-way ANOVA and paired student’s
>
Species distribution patterns
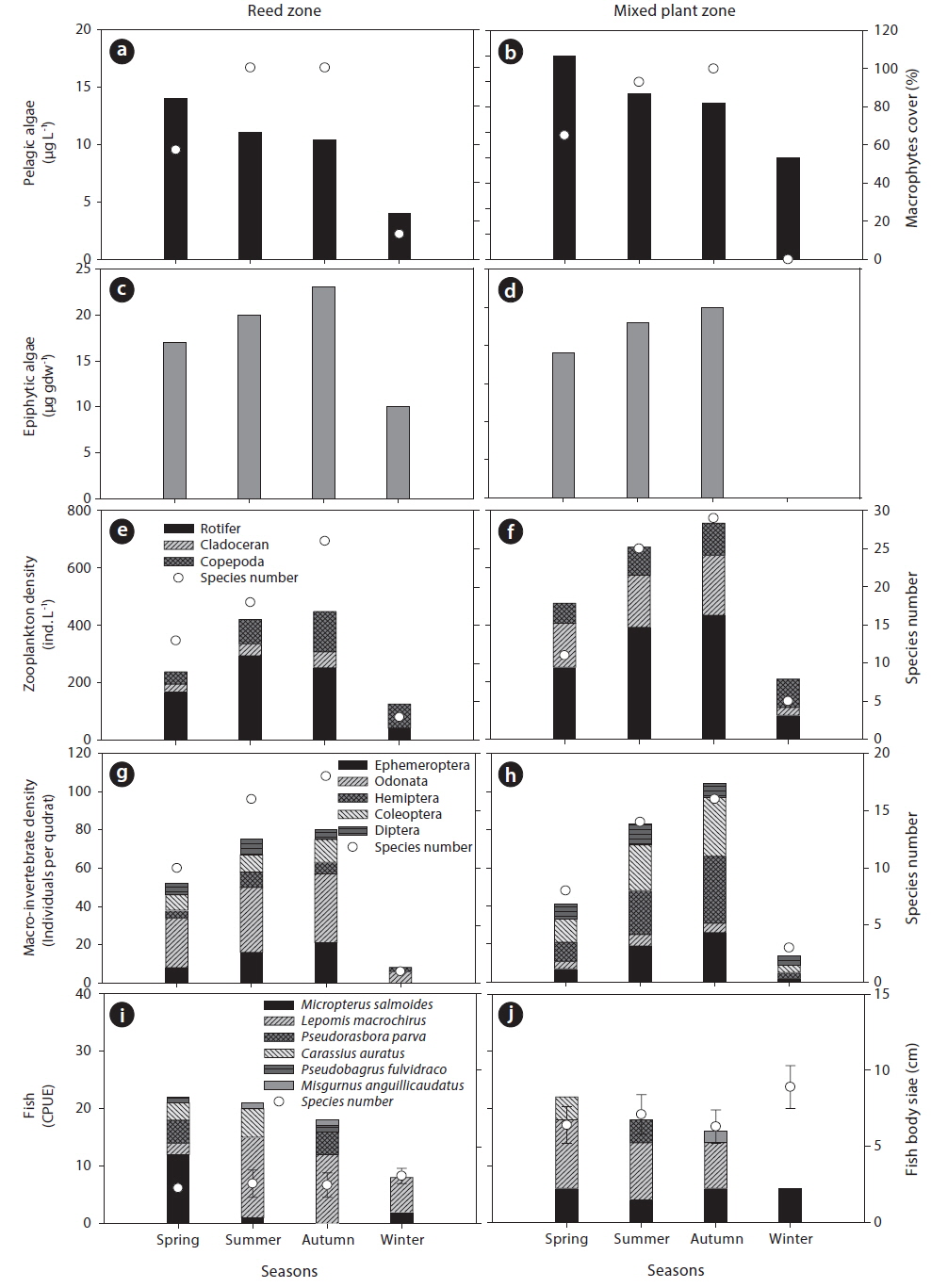
Aquatic organisms collected from the study site showed seasonal variation (Fig. 2). The density of pelagic algae was highest in spring and decreased as macrophyte cover increased. In contrast, the highest abundance of epiphytic algae was observed in autumn and was positively related to the seasonal increase of macrophyte cover. The seasonal distribution of zooplankton was also similar to epiphytic algal distribution. A total of 58 species of zooplankton were recorded during the study period; 41 species of rotifer, 14 species of cladocera, and 3 species of copepod. The mixed plant zone supported a greater density of zooplankton than the reed zone (Fig. 2e and 2f), and species diversity of zooplankton also showed the same seasonal pattern. The macro-invertebrate community was also more abundant in the mixed plant zone (Fig. 2i and 2j). However, the species composition of macro-invertebrates differed between the reed zone and the mixed plant zone. The reed zone had a larger abundance of macro-invertebrates from the order Odonata, but the mixed plant zone was dominated by Hemiptera and Coleoptera. Ephemeroptera had similar abundance in the reed zone and the mixed plant zone, and Diptera had the smallest abundance during the study period. The diversity of macro-invertebrate species was higher in the reed zone than in the mixed plant zone.
Fish abundance gradually decreased during study period, with the greatest abundance in spring. A total of 6 fish species were collected during the study period:
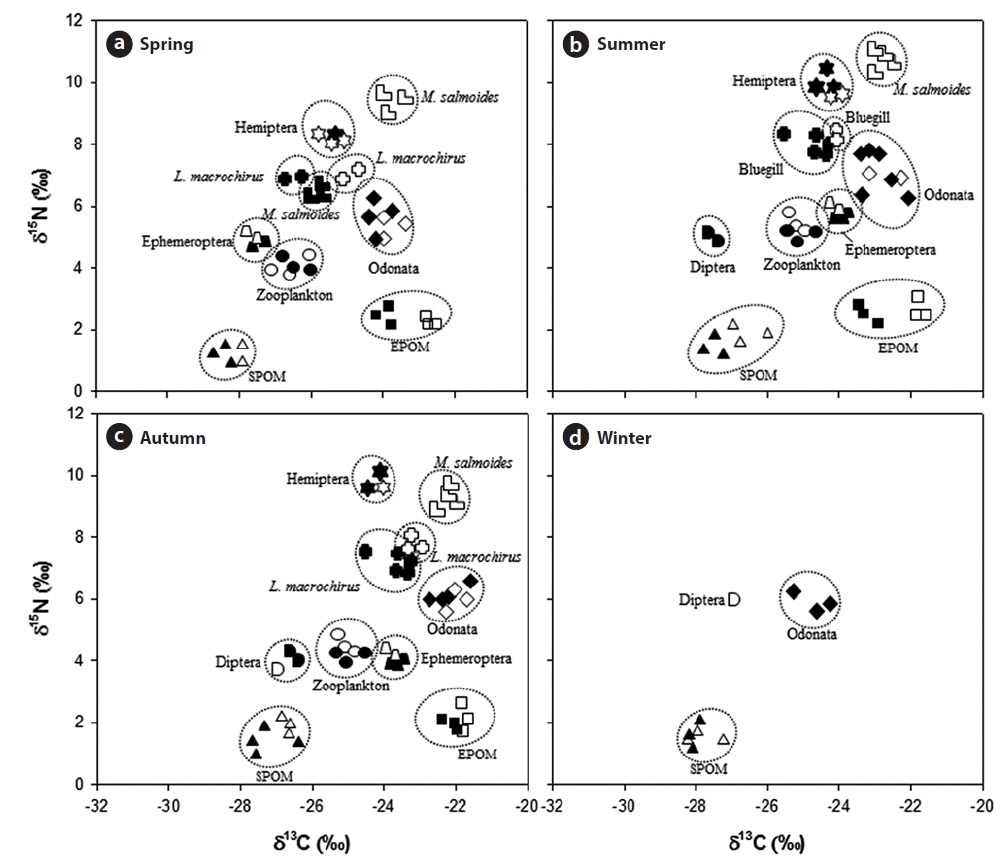
The stable isotope analysis showed trophic interactions among aquatic organisms in the Upo Wetland (Fig. 3 and Appendix 1 and 2). The δ13C and δ15N values of each aquatic organism were seasonally variable. The δ13C values for SPOM were significantly heavier in summer and autumn than in spring and winter (One-way ANOVA, df = 3,
Among aquatic animals, the δ13C values of Odonata were heavier than those of other aquatic organisms, and seasonally, Odonata had more δ13C in autumn. In contrast, the δ15N values of Odonata did not differ between seasons (One-way ANOVA, df = 3,
In general, 1‰ is used as fractionation coefficient for trophic step determination based on carbon isotopes and 2−3‰ for nitrogen isotopes, which allows relationships between predators and prey to be identified (France 1996, Lee et al. 2002). On the basis of this criterion, POM (SPOM and EPOM) is regarded as a primary food source for zooplankton and some macro-invertebrates. Among the macro-invertebrates observed here, Odonata mainly depended upon EPOM during the study period. EPOM was also observed to contribute to Ephemeroptera during the summer and autumn, whereas in spring Ephemeroptera were more dependent on SPOM. In contrast, Diptera were dependent solely on SPOM. Interestingly, Hemiptera tended to utilize macro-invertebrates and fish as food sources rather than SPOM and EPOM, and this relationship was stronger in summer and autumn. The collected Hemiptera was dominated by carnivorous species such as
However, the fish species depended on different food items in the reed zone and the mixed plant zone. The
In this study, we observed different seasonal distributions and habitat selection of aquatic organisms during study period. Pelagic- and epiphytic algae exhibited different seasonal distribution patterns, suggesting that they showed opposing relationship with the seasonal gradient of macrophyte cover. In general, epiphytic algae were strongly influenced on the seasonal development of macrophyte species due to qualitative and quantitative change of substrate surface of stem or root in accordance with season (Castro et al. 2007, Theel et al. 2008). The colonization by various macrophytes species increases the substrate surface for attachment of epiphytic algae, thus the macrophytes’ dominance can support high abundance of epiphytic algae. On the other hand, increased macrophyte cover can also cause a reduction in light penetration into the water through a shading effect. Thus, the density of photoautotrophs (i.e., phytoplankton) may decrease in the aforementioned environment. Empirical studies have demonstrated that phytoplankton compete with macrophytes, particularly for nutrients (Van Donk and Van de Bund 2002), and numerous studies have reported the absence or a lower density of phytoplankton where macrophytes are abundant (Sand-Jensen and Søndergaard 1981). In contrast, the epiphytic algae observed in the current study were composed mainly of diatoms, which are less sensitive to light. Bothwell (1988) reported that seasonal changes in insolation had no effect on the growth rates of epiphytic diatoms in freshwater ecosystems. Therefore, in the current study, it can be concluded that epiphytic algae was only influenced by the availability of macrophyte surface.
Zooplankton and macro-invertebrates also prefer macrophyte habitats with a complex structure. In general, zooplanktons are utilized as a primary food source for predators, such as fish (Lazzaro 1987). Thus, zooplanktons tend to be distributed in complex vegetated bed to avoid predators (Manatunge et al. 2000). The morphology of macrophytes species in vegetated bed is an important factor for determining habitat structure, and vegetated beds colonization by diverse macrophyte species form more complex structures. Therefore, it was assumed that the mixed plant zone would support a greater density of zooplankton than the reed zone. Moreover, among the zooplankton community, cladocera are well known to efficiently utilize macrophytes as a food source and for refuge (in particular, submerged macrophytes; Jacobsen et al. 1997), and they were frequently observed at high density in the mixed plant zone during the current study.
The abundance of macro-invertebrates also differed between the reed and the mixed plant zone. In particular, Odonata were more abundant in the mixed plant zone than reed zone. Typically, the abundance of Odonata is closely related to the richness of emergent macrophytes (Butler and de Maynadier 2008). Emergent plants seem to be more advantageous than other plant types, particularly for the emergence of adult Odonata. In contrast to emergent plants, floating-leaved or submerged macrophyte species are more flexible and easily agitated by water flow, which may encourage smaller and swimming macroinvertebrates to utilize these macrophytes. For example, upon hatching, the larvae of most damselflies forage, develop, and seek refuge among the submerged stems and leaves of aquatic macrophytes (Westfall and May 1996). This can also be applied to Hemiptera and Coleoptera (Barnes 1983). However, Hemiptera and Coleoptera have swimming patterns that differ from those of Odonata and need relatively extensive space for movement. Thus, they are likely to prefer the mixed plant zone compared to the reed zone.
Fish showed similar seasonal distribution patterns between the reed zone and the mixed plant zone. Among the collected fish species,
The stable isotope analysis showed different roles of fish between the reed zone and the mixed plant zone. In the reed zone,
Our results showed that macrophyte habitat segregation induced different species composition, abundance, and trophic interactions among aquatic organisms. The different structure observed between the vegetated habitats (reed and mixed plant zones) is clearly an important factor in elucidating the distribution of aquatic organisms. In the case of emergent macrophytes, they mainly have a firm stem submerged in the water, and thus they have a relatively less complex structure. In contrast, free-floating or submerged macrophytes commonly blend inhabits, and thus the mixed plants construct a complex structure in the water. Therefore, it can be concluded that the different structures between the two plant zones support different assemblages of aquatic organisms, strongly influencing the trophic interactions among the aquatic organisms. Some previous studies have reported that the most important role of macrophytes is to mediate interactions between prey and predators, and thus they may influence not only the survivability of prey individuals but also the continuous food supply for predators (Warfe and Barmuta 2004). Consequently, macrophyte species composition and vegetated bed structure strongly influence the distribution of aquatic organism assemblages and life stages of organisms recognized as an important factor to determine trophic-niche interaction.