Compared with freshwater ecosystems where cladocerans and rotifers are working as major grazers on primary producers, calanoid copepods are consisting major consumer group in marine ecosystems. Since marine calanoid copepods often show species-specific feeding behavior and feed not only phytoplankton but also protozoan including ciliates that graze the microbial food web components, the feeding of calanoid copepods can affect the microorganisms through the food web cascade as well as phytoplankton community through direct grazing (Zöllner et al. 2009). As eutrophication proceeds in estuaries and coastal areas, microbial food web components (e.g., bacteria, nanoflagellates, and ciliates) become key organisms in matter cycling (Katano et al. 2005, Hirose et al. 2008), and their interactions with herbivorous and omnivorous copepods have been considered as important linkage between microbial and herbivorous pathways in coastal marine ecosystems (Doi et al. 2008, Chang et al. 2009).
Temora Baird, 1850 is common genus inhabits the coastal marine area, and it often constitutes dominant grazer group. Owing to its limited ability to store energy, strong omnivorous feeding behavior depending on the surrounding food condition has been reported for a common Temora species, T. longicornis (Dam and Lopes 2003, Gentsch et al. 2009). The feeding behavior and food ingestion of genus Temora have been studied mainly for T. longicornis, and it has been suggested that the major food items include diatom, autotrophic dinoflagellates and ciliates. The feeding of T. longicornis plays a significant role in the transformation of particulate organic matter in the coastal ecosystems (Martynova et al. 2011). However, ingestion and food selection of Temora is strongly dependent on the concentration and size of available food items in the environments, and the information on the feeding behavior of this genus is still insufficient particularly with regards to 1) possible food item and range and 2) feeding behavior of other Temora species. Temora turbinata is widely distributed from tropical to temperate waters, and is often predominant in mesozooplankton communities of Korea and Taiwan (Jang et al. 2010). The feeding strategy of T. turbinata and the response of microbial food web components are still not sufficiently studied despite its frequent dominance and wide distribution (Wu et al. 2010).
In the present study, to elucidate the feeding behavior of T. turbinata and estimate its role in the pelagic food web, we carried out feeding experiment using the combinations of T. turbinata and natural food assemblages including phytoplankton and microbial food web components including bacteria, ciliates, and heterotrophic nanoflagellates collected from marine coastal area.
Feeding experiments were conducted twice during summer (August 31 to September 1 and September 22 to 23) in 2006, using natural plankton assemblage. Seawater containing plankton assemblage was collected from 5 m depth at Uchiumi Bay (32°55′ N, 132°30′ E), which is located at the coastal area of the Uwa Sea, Japan. Collected water was filtered through 70-μm-mesh net to remove mesozooplankton and drained into 0.6-L polycarbonate bottles. Mesozooplankton including calanoid copepods was collected from vertical net towing (50 m depth to surface) at the same location where natural plankton assemblage collection was made. Adult specimens of T. turbinata were sorted out from the collected mesozooplankton assemblage using a dissecting microscope and placed into 250-mL glass containers filled with seawater for 2-3 hours, and only active individuals were collected and used for the feeding experiments. As T. turbinata-treatment, 5 and 10 individuals of T. turbinata were introduced into prepared polycarbonate bottles containing seawater with natural plankton assemblage as ×1 and ×2 T. turbinata-treatment with 3 replicates, respectively. Three bottles without T. turbinata were used as the control. All bottles were placed on the mixing rotator to prevent permanent sink of phytoplankton, and kept dark under stable temperature (20℃).
Before the feeding experiment, subsamples were collected from the filtered water used for the experiment, to enumerate bacteria, heterotrophic nanoflagellates (HNF), ciliates, and phytoplankton abundances. After 24 hours, survival of T. turbinata in each treatment bottle was checked and active individuals were counted under light guide, and picked up from the bottle. Total of 100 mL of bottle water was collected from each bottle and fixed with glutaraldehyde for the numeration of bacteria and HNF. Bacterial density and HNF density were counted by using an epifluorescence microscope with ultraviolet excitation (Olympus, Tokyo, Japan) with excitation wave length 330 to 385 nm using the DAPI (Porter and Feig 1980) and primulin (Caron 1983) staining methods, respectively. Remained water was fixed with Lugol’s solution, and cell densities and species composition of phytoplankton were analyzed by using Burker-Turk counting chamber.
Clearance rates (F) of T. turbinata on bacteria, HNF, ciliates, and phytoplankton species were calculated according to Frost (1972) and Liu et al. (2005).
F (L Temora -1 day-1) = V( kc – kt)/z
where V is the volume of the incubation bottle (L), z is number of T. turbinata added to the incubation bottle and confirmed as active at the end of experiments, and kc and kt are the net or apparent prey growth rates in the controls and treatments, respectively, which are calculated by
k (day) = LN(Ce/C0)
for 24 hours incubations, where C0 is the initial concentration of prey, and Ce is the concentration of prey in the control and treatment bottles at the end of the incubation.
The densities of T. turbinata set as 5 and 10 individuals at the beginning of feeding experiments were average 5 and 8 individuals and 4 and 9 individuals, in the first and the second experiments, respectively.
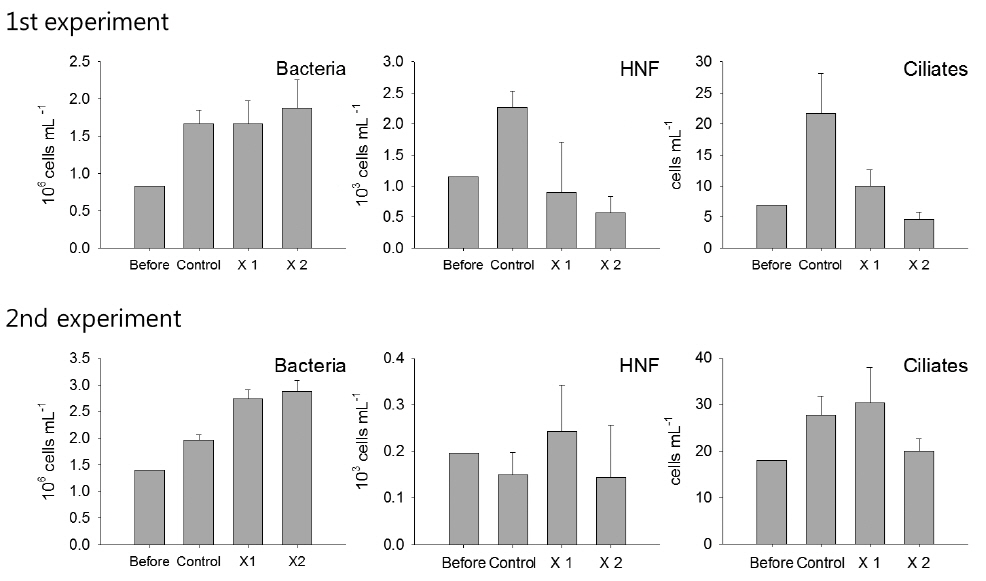
Among microbial food web components, bacteria showed no response against the feeding of T. turbinata, and no apparent difference was observed among the control and T. turbinata-treatments (ANOVA, P > 0.05). On the other hand, the abundances of HNF and ciliates decreased in T. turbinata-treatments according to increase of T. turbinata density in the incubation bottles in the first experiment. Their densities were significantly lower in T. turbinata-treatments compared with the control (ANOVA, P < 0.05), and the lowest densities were observed for ×2 T. turbinata-treatments where the grazer densities were doubled. However, such a pattern was not observed for both HNF and ciliates in the second experiment (Fig. 1).
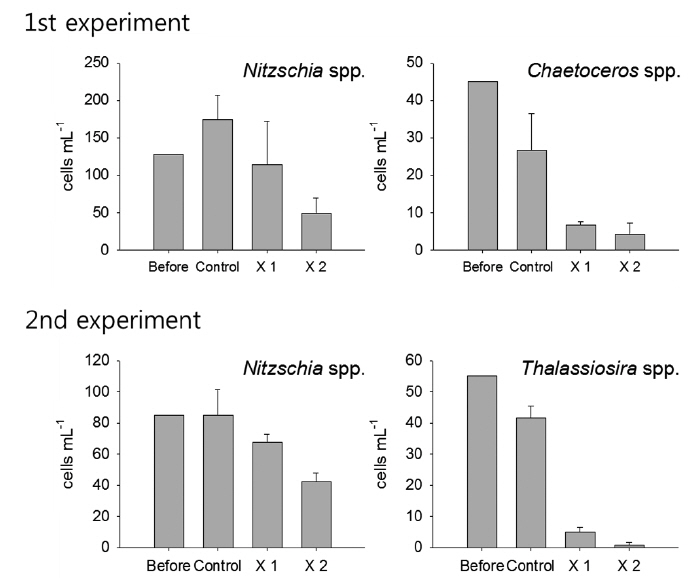
During the experiments, phytoplankton communities were dominated by diatom, but species compositions were different with dominances of Nitzschia spp. and Chaetoceros spp. in the first experiment while Nitzschia spp. and Thalassiosira spp. were dominant during the second experiment (Fig. 2). In the first experiment, although the density of Nitzschia spp. showed decreasing pattern in accordance with T. turbinata addition, the difference among the control and treatments was not significant due to the wide density variation in ×1 T. turbinata-treatments (ANOVA, P = 0.1683). On the other hand, abundance of Chaetoceros spp. decreased according to the T. turbinata addition near significantly (ANOVA, P = 0.06), and the density of Chaetoceros spp. dropped to near zero when 10 individuals of T. turbinata added.
In the second experiment, Nitzschia spp. showed same decreasing pattern with the first experiment with the lowest densities observed for higher T. turbinata densities (ANOVA, P = 0.06). The densities of Thalassiosira spp. drastically decreased with T. turbinata, and average remained cell density of ×2 T. turbinata-treatments was less than one cell mL-1 (ANOVA, P < 0.0001).
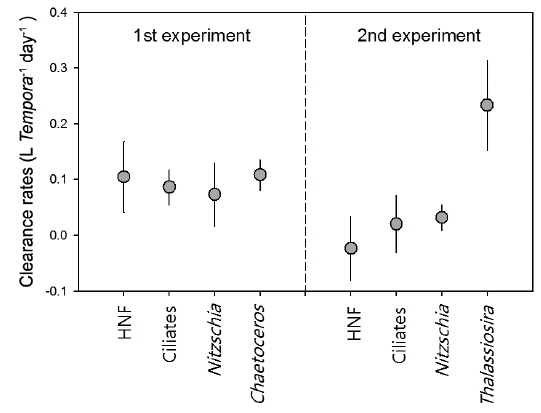
Clearance rate of T. turbinata for tested prey items was highest for Thalassiosira spp. as 0.23 ± 0.08 L Temora-1 day-1, showing similar range reported for Temora longicornis (Gentsch et al. 2009). During the first experiment, average (± standard deviation) clearance rate on Chaetoceros spp. was 0.11 ± 0.03 L Temora-1 day-1, which was higher than other prey species, but significant difference was not observed (Fig. 3). Compared with the first experiment where T. turbinata showed similar clearance rates for all prey types with the range between 0.07 and 0.11 L Temora-1 day-1, the rates were significantly low for all prey types in the second experiment except Thalassiosira spp. of which clearance rate was highest among tested prey types.
Temora turbinata has been known as suspension feeder, a typical algivore that prefers microscopic and non- or slow-moving phytoplankton. However, it has been suggested that T. turbinata is also able to exploit moving prey like ciliates (Wu et al. 2010). The present results suggest that T. turbinata has broad spectrum of prey selection from HNF to diatom, but Thalassiosira spp. seems to be the most preferable food item based on the highest clearance rate among prey assemblages. On the other hand, Nitzschia spp. showed the lowest clearance rates among diatom prey and can be considered as less-preferred diatom prey item.
Among microbial components, HNF are tiny organisms (from 5 to 20 μm) and it has been reported that HNF are not preferably consumed by calanoid copepods and often increase under grazing by calanoid copepods due to trophic cascade mediated by ciliates that mainly consume HNF (Bouvy et al. 2006). On the other hand, Pagano et al. (2006) have suggested the possibility of consumption of HNF by calanoid copepods because metabolic budgets for calanoid copepods suggested that consumption of phytoplankton could not balance their respiration needs. Insufficient energy can be satisfied by feeding upon HNF and/or detritus. In the present experiment, the response of HNF against T. turbinata feeding was prominent in the first experiment, and clearance rate for HNF showed similar range with other preferable prey items including diatoms and ciliates. However, in the second experiment, the response of HNF density and consequent clearance rates suggested no apparent feeding of T. turbinata on HNF. Additionally, T. turbinata showed high clearance rate only on Thalassiosira spp. The clearance rates on ciliates and Nitzschia spp. were lower than those measured in the first experiment. The results suggest that prey selection and consumption amounts can be modified by the presence of preferable prey item like Thalassiosira spp. in the environments. It has been suggested that genus Temora utilizes a broad range of food sources, but Temora often shows dietary shifts from omnivorous to a more herbivorous feeding mode with increase of phytoplankton abundance (Gentsch et al. 2009).
In conclusion, our results suggest that HNF are possible alternative prey item for the calanoid copepod T. turbinata, but their contribution as food sources can be available when preferable diatom species are scarce. The role of HNF and ciliates as “linkages” between microbial production and upper trophic levels can be modified by diatom species composition.
The present experiments were carried out under limited laboratory condition and prey assemblages during short period. Temora turbinata is one of dominant calanoid copepods in the East Asian marginal seas. Further studies considering species composition and size-fractionation of microbial components are necessary for the better understanding of prey selectivity of T. turbinata and its role as linkage between microbial production and upper trophic levels in coastal areas.