The biocide sodium hypochlorite (NaOCl) is widely used for controlling algal growth, and this application can be extended to marine environments as well. This study evaluates the biocidal efficiency and cellular toxicity of NaOCl on the harmful dinoflagellate Cochlodinium polykrikoides, with emphasis on pigment production and antioxidant enzyme activity. The test organism showed dose-dependent decrease in growth rate on exposure to NaOCl, and the 72 h EC50 was measured to be 0.584 mg L−1. NaOCl significantly decreased pigment levels and chlorophyll autofluorescence intensity, indicating possible detrimental effects on the photosystem of C. polykrikoides. Moreover, it significantly increased the activities of antioxidant enzymes, suggesting the production of reactive oxygen species in the cells. These data indicate that NaOCl exerted deleterious effects on the photosynthetic machinery and induced oxidative damage in the dinoflagellate and this biocide could be effectively used for the control of algal blooms.
Sodium hypochlorite (NaOCl) is used as a disinfectant or a bleaching agent, and it is the most commonly used biocide in the drinking water industry and swimming pools (Hahn et al. 2005). NaOCl is generally used because it is cost efficient, readily available and easy to handle (Peker et al. 2014). NaOCl reacts with water to form hypochlorous acid (HOCl) (White 2010), which interferes with protein unfolding, inhibits glucose oxidation (Winter et al. 2008), and depletes adenine nucleotides (Rosen et al. 1998), thereby causing physiological and biochemical damages to the exposed organisms (Vitro et al. 2005). Moreover, since HOCl is a reactive oxygen species (ROS) (Halliwell 2006), it has the potential to be used for the removal of harmful algal blooms (HABs) in marine environments. Application of NaOCl to HAB species may result in cellular damages and reduced cell division (Estrela et al. 2002). However, effects of NaOCl on the cellular responses of HABs have not been studied sufficiently, in particular, with emphasis on physiological and biochemical aspects.
Aerobic organisms generate different ROS such as super oxide anion, hydrogen peroxide, hydroxyl radical, and hypochlorous acid through the reduction of molecular oxygen (Spickett et al. 2000). Low to moderate levels of ROS serve as signaling molecules for altering gene expression and for modulating the activities of stress-related proteins (Sharma et al. 2012). Various external factors such as biocides, light, temperature, and pH variations stimulate ROS production (Sharma et al. 2012). High concentrations of ROS lead to peroxidation of lipids, oxidation of proteins, damage to nucleic acids, etc. (Mishra et al. 2011). However, cells can protect themselves against such oxidative damage through well-organized antioxidative systems that include several low-molecular-weight compounds (e.g., carotenoids [CAR]) and antioxidant enzymes (Tripathi et al. 2006). For example, in cells, super oxide dismutase (SOD) acts as the first line of defense against ROS (Ken et al. 2005). Other antioxidant enzymes such as catalase and peroxidase are reported to counteract oxidative stress in both higher plants and microalgae (Wang et al. 2012).
In the present study, we determined the efficiency of the biocidal agent NaOCl in controlling the growth of the harmful dinoflagellate
>
Experimental organism and toxicity assay
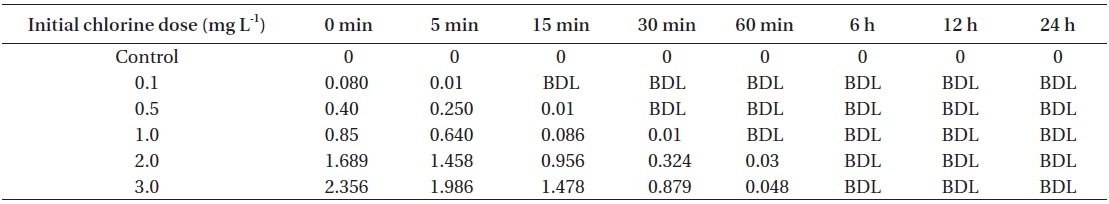
NaOCl (Cat. No. 425044; Sigma, St. Louis, MO, USA) was commercially obtained. The stock solution (1,000 mg L−1) was prepared by diluting the commercially obtained solution with autoclaved distilled water (ADW) for which the chlorine demand was previously determined to be below the detection limit (<0.01 mg L−1). Nominal doses of chlorine 0.1, 0.5, 1.0, 2.0, and 3.0 mg L−1 were prepared by diluting the stock in ADW. Free chlorine concentration (concentration of hypochlorous acid and hypochlorite ion) was spectrophotometrically determined by N,N-diethyl-phenylene-diamine (DPD) at 515 nm (Clesceri et al. 1998). The chlorine demand of the f/2 medium was determined to be 1.2 mg L−1 (5 mg L−1 of chlorine was dosed tothe medium in triplicate and the contact time was 60 min to determine the chlorine demand).
Doses of NaOCl adjusted according to chlorine demand, as described above, were added to 200 mL of
>
Cell observations and the median effective concentration (EC50)
Cell counts in each test flask were determined using a plankton-counting chamber (HMA-S6117; Matsunami Glass, Osaka, Japan) and were plotted against time of exposure to NaOCl.
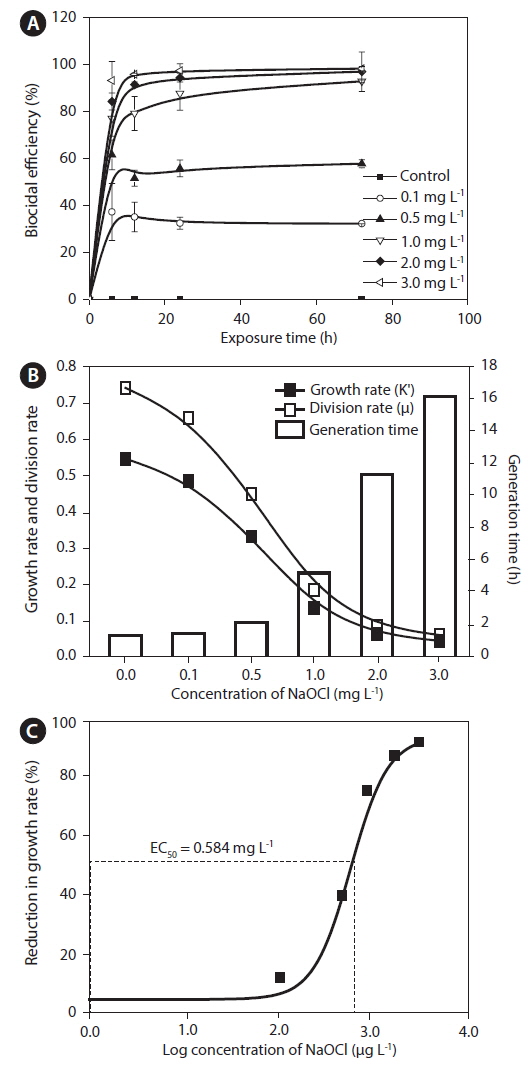
The growth rate was calculated based on the following equation (Levasseur et al. 1993): Growth rate (K′) = Ln (N2 / N1) / (t2 − t1), where N1 and N2 are biomass at time 1 (t1) and time 2 (t2), respectively. In addition, division rate (μ) and generation time were calculated as follows: Divisions per day = K′ / Ln2, and generation time = 1 / (division day−1), respectively.
Biocidal efficiency was calculated based on percent inhibition, following the recommendation by Organization for Economic Cooperation and Development (2011). The percent inhibition was calculated based on the following equation: % I = (μC − μT / μC) × 100, where % I = percent inhibition, μC = mean value of growth in the control, and μT = mean value of growth rate in the treated samples.
Levels of chlorophyll
EC50 at 72 h and the percentile inhibition were calculated as recommended in Organization for Economic Cooperation and Development testing guidelines (Organization for Economic Cooperation and Development 2011). The EC50 values were estimated using a sigmoidal dose-response curve and plotted using Origin ver. 8.5 (MicroCal Software Inc., Northampton, MA, USA).
>
Biochemical (lipid peroxidation and SOD) assays and statistical analysis
Lipid peroxidation was measured according to the method of Heath and Packer (1968). The cells were harvested by centrifugation at 4,217 ×g for 10 min, and then 2 mL of 10% trichloroacetic acid (TCA) was added to the pellet. The cells were homogenized using a Teflon pestle tissue homogenizer in ice. The tube was then placed in a water bath at 40℃ for 5 min, followed by a modification of the method described by Soto et al. (2011). The mixture was centrifuged at 4,217 ×g for 10 min. An equal volume of 0.25% thiobarbituric acid freshly prepared in 10% TCA solution was added to the supernatant. The tube was heated at 95℃ for 30 min in a water bath. The mixture was then cooled to room temperature and centrifuged for 10 min at 4,217 ×g. The absorbance of the solution was measured at 532 nm. Results were expressed as micromoles of malondialdehyde (MDA) per 104 cells.
SOD was assayed according to the method of Beauchamp and Fridovich (1971). Algal cells were harvested by centrifugation at 4,217 ×g for 10 min, and then 5 mL of 100 mM dihydrogen phosphate buffer was added to the algal cell pellet. The cells were homogenized using a Teflon pestle tissue homogenizer in ice. Next, the tube was placed in a water bath at 40℃ for 5 min using a method modified from Soto et al. (2011). The mixture was centrifuged at 4,217 ×g for 10 min. To the supernatant, 2.6 mL of the reaction mixture (0.5 M phosphate buffer, 130 mM methionine, 750 μM Na2EDTA, and 20 μM riboflavin) were added. The tubes were incubated in light (65 μmol photons m−2s−1) for 30 min. The absorbance was read at 560 nm. One unit of SOD (U) was defined as the amount of enzyme resulting in 50% inhibition of photochemical reduction of nitroblue tetrazolium. SOD levels were represented as U per 104 cells (U 10−4 cells−1).
All data presented here are mean values obtained from experiments performed in triplicates. One-way analysis of variance (ANOVA) with post hoc Dunnet’s test using Graphpad InStat (Graphpad Software Inc., San Diego, CA, USA) was used for comparisons between untreated and treated cultures. p < 0.05 was accepted as significant. In addition, correlation between cell counts, Chl
>
Effects of NaOCl on growth and division rates
[Table 1.] Total residual oxidant levels at different time intervals
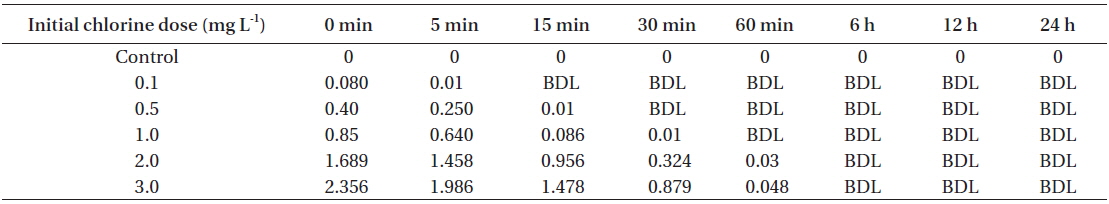
Total residual oxidant levels at different time intervals
>
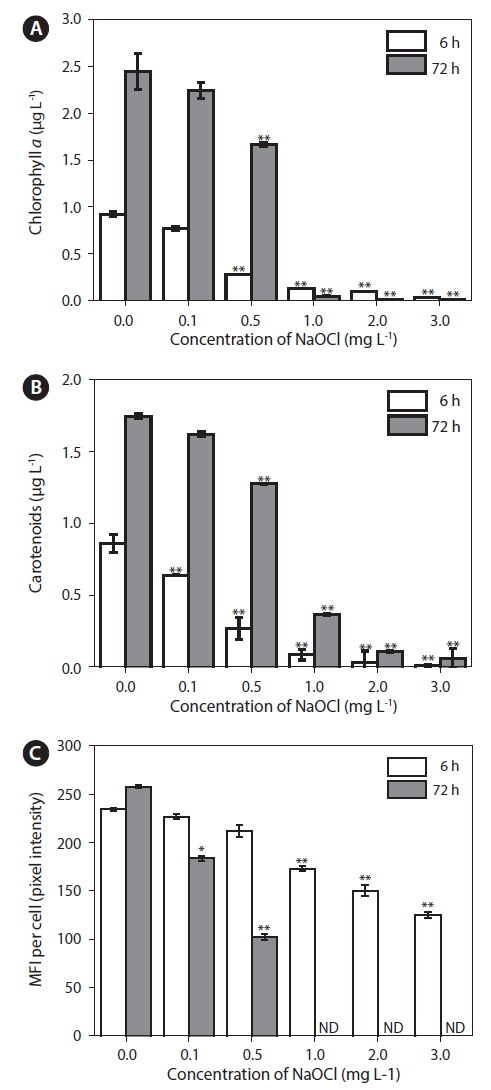
Effects of NaOCl on Chl a, CAR, and CAF levels
Chl
Physiological activity of
>
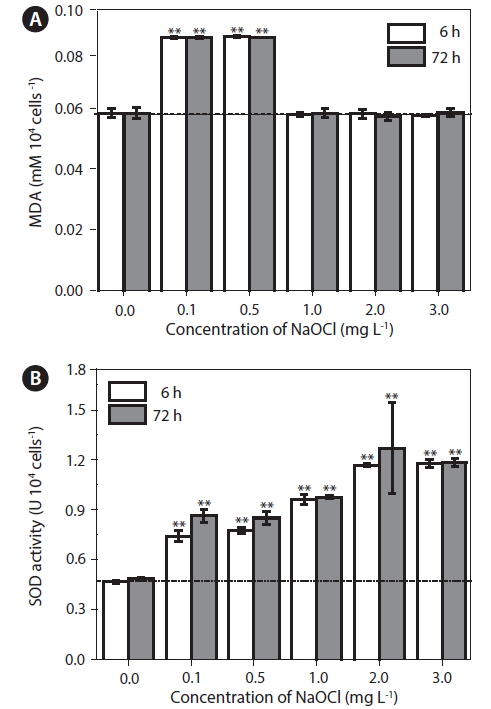
Effects of NaOCl on antioxidant enzyme activity
There was a significant (p < 0.05) increase in lipid peroxidation at lower doses (0.1 and 0.5 mg L−1) of NaOCl after 6 h of exposure (Fig. 3A), and a similar trend was observed up to 72 h. The MDA levels increased from 0.058 to 0.083 mM per 104 cells. However, cultures exposed to 1.0, 2.0, and 3.0 mg L−1 of NaOCl did not showed significant variation (p > 0.05) in lipid peroxidation throughout the experiment.
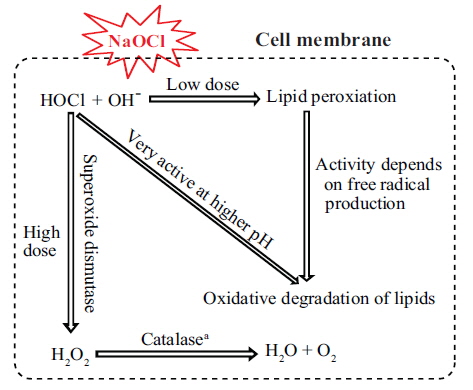
In cultures exposed to 0.1 and 0.5 mg L−1 NaOCl, after 6 h, SOD activity increased (p < 0.05) to approximately 4-5 times that in the unexposed controls and the increase continued up to 72 h of exposure (Fig. 3B). In addition, for cultures exposed to 1.0, 2.0, and 3.0 mg L−1 of NaOCl the SOD activity increased to approximately 7-10 times that in the control. Fig. 4 shows a schematic view of the variation in lipid peroxidation and SOD activity after exposure to NaOCl, as observed in this study.
NaOCl, a disinfectant and a biocide, affects the physiological and biochemical processes of organisms such as bacteria and fungi by damaging their intracellular molecules (Vitro et al. 2005). Here, we evaluated the applicability and physiological effects of the biocide for the removal of the harmful dinoflagellate
Measurement of CAF has been reported to be a rapid, sensitive method for assessing the physiological status of microalgae (Nancharaiah et al. 2007), because autofluorescence enables discrimination of damaged and undamaged cells (Sato et al. 2004). Microalgae exhibit auto-fluorescence because of the presence of photosynthetic pigments (Trampe et al. 2011). In the present study, CAF as MFI per cell (
The biocidal effect of NaOCl involves the action of free radicals generated by a Fenton-type reaction and the formation of HOCl (Bajszár and Dekonenko 2010), which lead to oxidative stress (Leichert et al. 2008). The generation of free radicals results in the oxidative degradation of lipids, which is the main target of oxidative stress, as revealed by the increase in lipid peroxidation activity (Cabiscol et al. 2000). In the present study, we observed an increase in lipid peroxidation activity after exposure to low doses of NaOCl (Fig. 3A). This result might be attributed to the involvement of free radicals at the cellular level (Li et al. 2002). It was supported by previous works that trace amount of H2O2 (about 10−8 M) are always present in water as a result of natural radiolysis (Arnhold et al. 1991); HOCl reacts with H2O2 according to a Fentontype reaction forming hyroperoxides, and thus initiating lipid peroxidation (Panasenko et al. 2013). HOCl induced lipid peroxidation is completely blocked by scavengers of free radicals (e.g., taurine), when the lipid peroxidation reaches saturation (Spickett et al. 2000). Moreover, pH was also found to have an influence in HOCl induced lipid peroxidation (Panasenko et al. 1997). The decrease in lipid peroxidation activity at high NaOCl doses in the present study could be due to increased production of free radicals.
In aerobic organisms, the termination of lipid degradation is enhanced by several molecules (e.g., SOD, catalase, and peroxidase) that remove free radicals from the system. For example, SOD is involved in the dismutation of superoxide into O2 and H2O2 (Shin et al. 2005). In the present study, we observed a significant increase in the level of SOD after exposure to higher concentrations of NaOCl (Fig. 3B). A previous study by Li et al. (2005) also reported increase in SOD activity when the marine alga
In conclusion, our findings show that NaOCl reduces the growth rate and pigment levels of the test dinoflagellate