Red algal parasites are common within red algae and are mostly closely related to their hosts, but have a reduced habit. In the past, red algal parasites, due to their reduced morphology, have been given distinct generic names, even though they are often phylogenetically nested in their host's genus. This is a problem nomenclaturally for maintenance of a taxonomy based on monophyly. This study investigates the morphology, genetic variation and distribution of an undescribed red algal parasite growing on its host Rhodophyllis membranacea, widely distributed throughout New Zealand. Microscopy, molecular markers (plastid, mitochondrial, nuclear), and herbarium investigation were used to investigate this species. The parasite is widely distributed throughout New Zealand. All molecular markers clearly show that the parasite is almost identical to the host, even though morphologically quite distinct from members of the host genus. We believe that to maintain monophyly of Rhodophyllis the parasite should be described as a new species of Rhodophyllis, Rhodophyllis parasitica sp. nov. We also recommend that in order to maintain generic monophyly most red algal parasite genera should also be transferred to their host genus.
Red algal parasites are common on other red algae and have been described from several orders within the Florideophyceae (Goff 1982), a few molecular-based phylogenetic studies have focused on a handful of species (Goff et al. 1997, Zuccarello et al. 2004, Ng et al. 2013), with new species still being described (Kim and Cho 2010).
The morphological characteristics traditionally used to determine if a red alga is a parasite include: 1) the penetration of the parasite beyond the superficial cells of the host (Setchell 1918); 2) reduction of the parasite thallus; and 3) the loss of colour (Wynne and Scott 1989). Another important character are the presence of secondary pit connections (2nd PC) formed between parasite and host cells (Goff 1982). 2nd PC have been observed in nearly all described red algal parasites studied (e.g., Fredericq and Hommersand 1990, Goff and Zuccarello 1994, Goff and Coleman 1995).
Molecular studies are helpful in determining the evolutionary relationship between host and parasite (Zuccarello et al. 2004). Gene sequences of plastid DNA have shown that they are mostly identical between hosts and parasites, which suggests that red algal parasite retain the host plastids (Goff and Coleman 1995). Comparison of mitochondrial and nuclear sequences between hosts and parasites has shown differences between them, which suggests that the red algal parasite retains its own mitochondria and nuclei (Goff and Coleman 1995).
Several studies have shown that red algal parasites are often more closely related to their host than the hosts are to other species in the host genus (e.g., Goff et al. 1996, Kurihara et al. 2010). In all these cases, a distinct genus name for the parasite has been retained, rendering the host genus paraphyletic. A strict monophyletic (holophyletic) classification system is supported by many systematists but maintaining paraphyly (for evolutionarily innovative lineages) also has backers. The arguments about maintaining strict holophly is on-going (Hörandl and Stuessy 2010) and may depend on the purposes of a classification system.
There are four reported red algal parasites in New Zealand.
We describe a new red algal parasite from New Zealand growing on
List of all samples of Rhodophyllis membranacea and its parasite collected and used in molecular analysis
Parasite tissue with surrounding host tissue was removed, fixed overnight in 2% glutaraldehyde in phosphate buffer (0.1 M, pH 6.8) in 50% seawater. The tissue was then washed three times at intervals of 10 min with phosphate buffer (0.1 M, pH 6.8) in 50% seawater. The tissue was dehydrated in an ascending ethanol-water series of 20, 40, and 60% ethanol and three times with 70% ethanol for 15 min each. The fixed samples were stored in 70% ethanol at 4℃ until embedding in glycol methacrylate (JB-4; Sigma-Aldrich, St. Louis, MO, USA) following the manufacturers instructions. The embedded stubs were covered with parafilm and stored in a desiccator until hardened. The embedded tissue was sectioned on a microtome (860 Model; American Optical Corporation, Southbridge, MA, USA). Sections were 10 µm thick and carefully transferred from the surface of distilled water to a microscopic slide after they had fully spread in the water. Slide were then dried and stored.
All microscopic slides were either stained with 1.0 µg mL−1 DAPI in McIlvaine buffer (pH 4.1) or 1% acidified aniline blue solution. DAPI was used to stain nuclei and Aniline blue was used as a general cytological stain. The microscopic slides were examined under a fluorescence microscope (Olympus AX-70) with integrated camera (Olympus DP-70; Olympus, Tokyo, Japan) and images were taken using either DP Controller (Olympus) imaging software or Stream Enterprise software (Olympus).
DNA from fresh, or silica gel dried samples, was extracted with 5% Chelex following Zuccarello et al. (1999) (Table 1). Two mitochondrial markers (
Amplification reaction (30 µL) were performed with the following final concentrations: 1× buffer (BioTherm; Genecraft, Ludinghausen, Germany), 0.2 mM dNTP’s, 2.5 mM MgCl2, 0.04% BSA (Sigma), 0.25 pmol of each primer, 1 U
Forward and reverse sequences were assembled and edited in Geneious. The edited sequences were aligned using MAFFT alignment using the default parameters. The alignment was checked and realigned by eye. The alignment of
All herbarium samples of
Morphology of the
Habitat and seasonality. The red algal parasite growing on
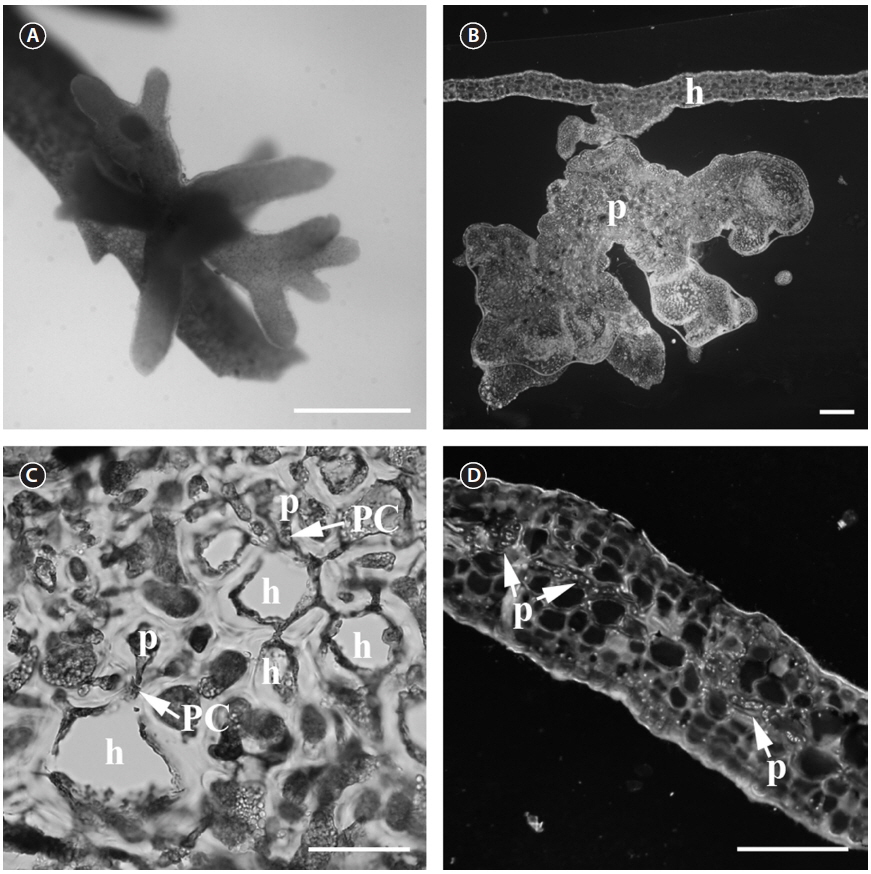
Thallus. The parasite (Fig. 1A) is less pigmented than the host (light reddish), which makes the parasite easy to spot. The parasite thallus is on average 2.5 × 3 mm in size and consists of a radiating cluster of terete, smooth branches that often terminate bipinnately or remain unbranched (Fig. 1A). The parasite has a single base attached to the host surface and a short stipe that branches distally (Fig. 1B). The branches are not flattened as in
Vegetative structures. The thallus area, which connects the host and parasite cells, consists of elongated cells of various sizes. This contact area also contains embedded host cells between parasite cells. The parasite cells form secondary pit connections with host cells (Fig. 1C). Secondary pit connections can be found between large cortical host cells and small parasitic cells in the contact area. The small cells cannot be host cells as the host has a very regular cell distribution pattern. The vegetative structure of
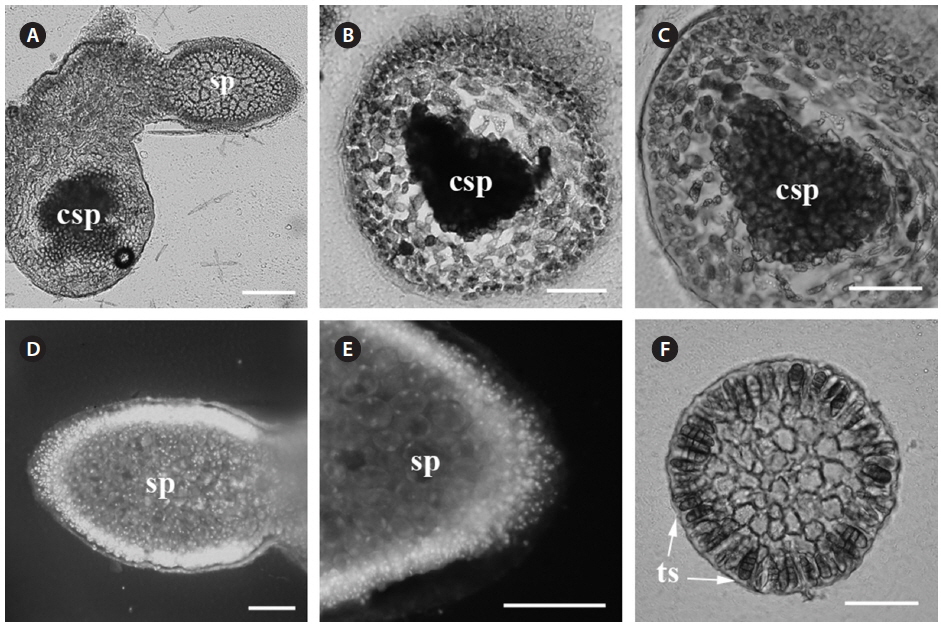
Reproductive structures. Carposporophytes, spermatangia and tetrasporangia were observed. The parasite has bisexual gametophytes (Fig. 2A).
Carposporophyte. The mature carposporophyte (Fig. 2A-C) contains oval carposporangia with an average size of 20 µm (n = 5), and are located marginally on the branches of the parasite (Fig. 2A). Carposporophytes are 230 × 330 µm (n = 3) and surrounded by a pericarp. The pericarp is approximately 5-10 cells thick (200 µm; n = 3) (Fig. 2C).
Male gametophyte. Spermatangia are in dense patches on the surface of the parasite thallus (Fig. 2D). Spermatia are round and less than 10 µm in diameter (Fig. 2E).
Tetrasporophyte. Tetrasporangia are scattered and numerous on the surface. Tetrasporangia are zonately divided (Fig. 2F). Tetrasporangia are pigmented and of an average size of 48 × 20 µm (n = 10).
>
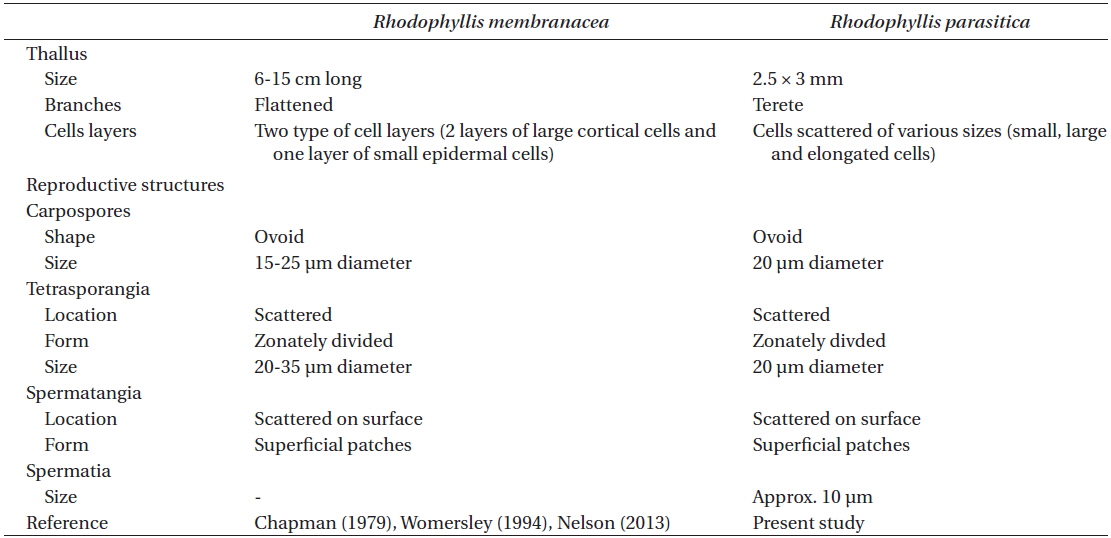
Comparison of morphological characters between host and parasite
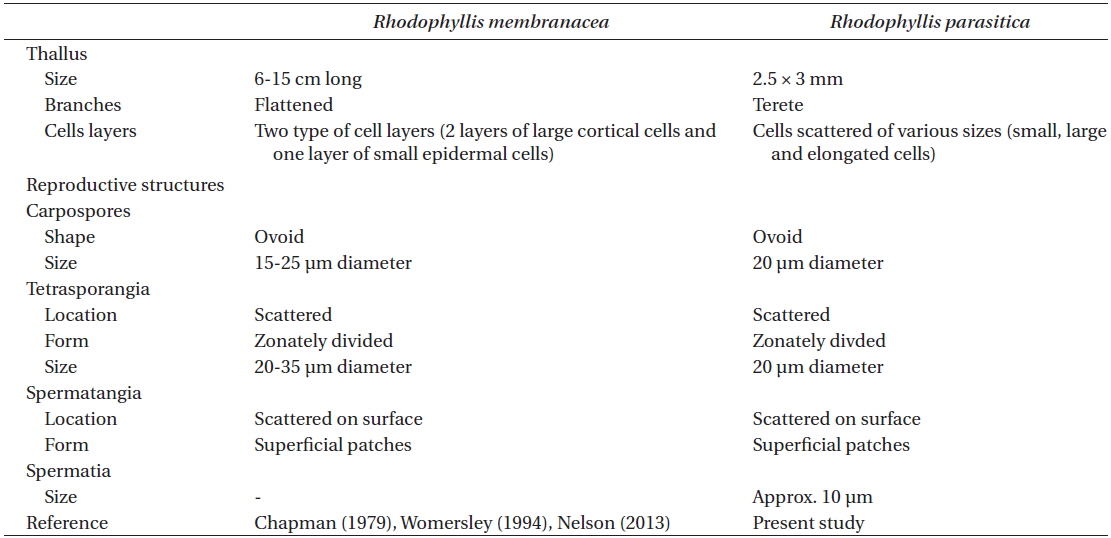
Comparison of vegetative and reproductive structures between Rhodophyllis membranacea and its parasite
>
Phylogeny of Rhodophyllis parasite
ITS2. The 512 basepairs (bp) ITS2 alignment contained 22 samples of
RuBisCo spacer. The RuBisCo spacer alignment contained 4 samples of
>
Distribution of the Rhodophyllis parasite
The Te Papa herbarium collection contained over 60 specimens of
[Table 3.] Te Papa vouchers of Rhodophyllis membranacea with red algal parasites on them
Te Papa vouchers of Rhodophyllis membranacea with red algal parasites on them
Our genetic evidence clearly shows that this parasite is closely related to its host. It is almost indistinguishable genetically with the markers used that have been used to distinguish species in other red algae (e.g.,
Since the level of genetic similarity between the parasite and host is less than expected between two species of red algae, it is likely that the parasite is more closely related to its host than
Due to the morphological and genetic similarity of red algal parasite and host, it has been suggested that the parasite evolved from its host (Goff et al. 1997, Blouin and Lane 2012, Ng et al. 2013). For example, the parasite
Red algal parasites have traditionally been placed in a separate genus from their host since their first description (Fan and Papenfuss 1959, Lee and Kurogi 1978). Red algal parasites have special morphological characters such as their small size and reduced pigmentation due to their parasitic lifestyle, but reproductive characters suggested their close similarity to their host in many cases (Goff 1982). These morphological similarities suggested that parasites should be classified at least within the same family as their host. Molecular methods have improved our understanding of evolutionary relationships in organisms. Phylogenetic nomenclature proposes that modern classification should reflect phylogenetic relationships and not support paraphyly (De Queiroz and Gauthier 1994) even suggesting the removal of nomenclatural ranks. Often an emphasis on monophyly as a criterion for rank assignment takes precedence over paraphyly that could highlight evolutionary novelty (Hörandl 2007, Hörandl and Stuessy 2010). The novel origin of these parasites from their hosts, an evolutionary relationship that is common in red algal parasites but unknown in other parasitisms, should be reflected in the taxonomy over the changes in morphology due to the parasitic mode. The incorporation of the parasites into the host genus also changes the morphological circumscription of the host genus. We feel that this is not a serious issue as the genus can be amended to include “and the parasites derived from it,” but this needs to be tested as some parasites are not so closely related to their hosts (Zuccarello et al. 2004). The first example to follow this practice is the transfer of
Another character used in the description of red algal parasites was the presence of sporophyte and gametophyte of the parasite on the same host plant (Wynne and Scott 1989). At this point of the study, while gametophytes and sporophytes were collected from the same location we did not note if they are from the same host plant. Further investigation of this character needs to be done to fulfil designation of all previous mentioned characters of red algal parasites.
The parasite penetrates beyond the superficial cells of
The three ITS2 ribotypes and
The red algal parasite of
We describe this parasite on
>
Rhodophyllis parasitica Preuss et Zuccarello sp. nov.
Diagnosis. Thalli coloured (light reddish), size 2.5 × 3 mm, branches mostly irregular to bipinnately branched, and terete with smooth surface and blunt branch tips. Bisexual gametophyte. Carposporophyte 230 × 330 µm, surrounded by pericarp, containing oval carposporangia. Carposporangia 20 µm in diameter, located marginally on branches. Spermatangia 10 µm in diameter, in dense patches on thallus surface. Tetrasporangia scattered on surface, zonately divided, 48 × 20 µm. Parasitic on
Type locality. RPPZ0212; 41°20′33″ S, 174°47′6″ E; growing on
Holotype. WELT A032962; collected by M. Preuss.
GenBank accession numbers. COI: KM407523; ITS2: KM407520;
Etymology. The specific epithet refers to the parasitic lifestyle of this red alga.