Inflammation is a defense response in a wide variety of physiological and pathological processes caused by stress, injury and infection (Kim et al. 2010). This process contributes to impairment on the immune system when persisted for a long period of time as activated macrophage produces toxic factors. Macrophages activated by lipopolysaccharide (LPS) or interferon gamma secrete nitric oxide (NO), cyclooxygenase-2 (COX-2) and other intermediates to destroy the remaining microorganisms in the inflammation response. In particular, exposure to LPS activates macrophage to secrete pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 through the expression of inducible nitric oxide synthase (iNOS) and NO whereas prosPhlorotaglandins production is mediated by COX-2 (Ulevitch and Tobias 1999, Akira et al. 2001). Moreover, this process activates a set of extracellular stimuli-dependent signal transduction cascades such as the mitogen-activated protein kinases (MAPKs) pathway (Akira et al. 2001). MAPK pathway, such as the extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase/stress-activated protein kinase (JNK) signaling pathways, regulate inflammatory and immune responses and are known to be involved in LPS-stimulated iNOS and COX-2 expression in macrophages (Kim et al. 2010).
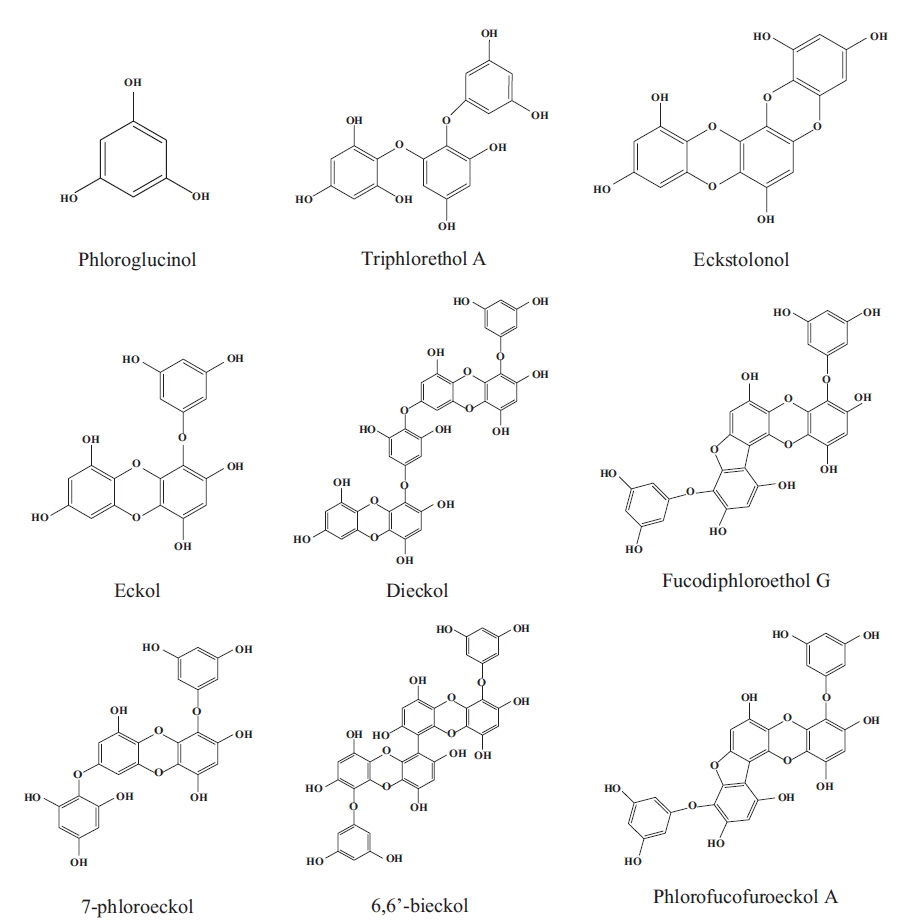
Marine algae or seaweeds are classified based on their colors as red algae (Rhodophyta), brown algae (Phaeophyta), and green algae (Chlorophyta). Red algae are more well known because 4,500 species have dominated along the coastal and continental shelf areas of tropical, temperate and cold-water regions for thousands of years, whereas the remaining algae include 1,000 species of brown and 900 species of green algae (Athanasiadis 2003). Red algae are ecologically significant as primary producers and providers of the structural habitats for other marine organisms, and their important role is in the primary establishment and maintenance of coral reefs. Moreover, the high level of minerals, vitamins, essential amino acids, indigestible carbohydrates and polyphenols, as well as their bioactive metabolites, in red and their relatively easy cultivation makes them attractive (Xu et al. 2003, Kelman et al. 2012) (Fig. 1). Therefore, many maritime countries have used red algae as a source of food, for industrial applications, a fertilizer and for medicinal purposes due to their biological activities.
The red alga
Dulbecco’s modified Eagle’s medium (DMEM), penicillin/ streptomycin, fetal bovine serum (FBS) and the other materials required for culturing cells were purchased from Gibco BRL Life Technologies (Grand Island, NY, USA). LPS of
>
Cell culture and viability assay
RAW 264.7 macrophages were purchased from the Korea Cell Line Bank (Seoul, Korea) and maintained in a 5% CO2 humidified atmosphere and at 37℃ in DMEM supplemented with 10% heat-inactivated FBS, 100 μg mL−1 streptomycin, and 100 U mL−1 penicillin. The cell viability was determined by the MTT reduction assay as described by Hansen (Hansen et al. 1989). RAW 264.7 macrophages plated on 96-well plates were pre-incubated and subsequently treated with LPS (1 μg mL−1) coupled with aliquots of the CJE at 37℃ for 24 h. The MTT stock solution (50 μL) was then added to each well to obtain a total reaction volume of 250 μL. After 4 h of incubation, the plates were centrifuged (800 ×g, 5 min), and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 μL of DMSO, and the absorbance was measured with an enzyme-linked immunosorbent assay plate reader (BioTek Instruments, Winooski, VT, USA) at 540 nm.
The NO production in the culture supernatants was measured by the Griess reaction as described previously by Coker and Laurent (1998). After a 24 h pre-incubation of the RAW 264.7 macrophages with LPS (1 μg mL−1), the exquantity of nitrite accumulated in the culture medium was measured as an indicator of NO production. In brief, 100 μL of the cell culture medium was mixed with 100 μL of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediaminedihydrochloride in 2.5% phosphoric acid), the mixture was incubated at room temperature for 10 min, and the absorbance at 540 nm was measured using a micro-plate reader (BioTek Instruments). Fresh culture medium was employed as a blank in every experiment.
>
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
The total RNA from the macrophages treated with LPS in the presence or absence of the CJE was extracted using the TRIzol reagent. Equal amounts of RNA were used for each cDNA synthesisreaction. Adjusted oligodT primers (10 μM) with DEPC were added, and the mixture was then incubated for 5 min at 70℃ and then snap-cooled on ice. The isolated mRNA was then used to synthesize cDNA according to the manufacter’s instruction (Promega, Madison, WI, USA). The PCR was performed in an automatic Whatman thermocycler (Biometra, Kent, UK). Single-stranded cDNA was amplified by PCR using specific primers. The primer sequences used to amplify the desired cDNA fragment were as follows: COX-2 forward and reverse primers: 5′-GGGGTACCTTCCAGCTGTCAAAA TCTC-3′ and 5′-GAAGATCTCGCCAGGTACTCACCTG-3′; iNOS forward and reverse primers: 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′; IL-1β forward and reverse primers: 5′-ATGGCAACTGTTCCTGAACTCAACT-3′ and 5′-TTTCCTTTCTTAGATATGGACAGGAC-3′; IL-6 forward and reverse primers: 5′-AGTTGCCTTCTTGGGACTGA-3′ and 5′-CAGAATTGCCATTGCACAAC-3′; TNF-α forward and reverse primers: 5′-ATGAGCACAGAAAGCATGATC-3′ and 5′-TACAGGCTTGTCACTCGAATT-3′ and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward and reverse primers: 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ and 5′-CATGTAGGCCATGAGGTCCACCAC-3′. The following PCR conditions were applied for all amplifications by 30 cycles of 94℃ for 30 s (denaturing), 57℃ for 30 s (annealing), and 72℃ for 30 s (primer extension). The resulting cDNA was separated by electrophoresis on a 1% agarose gel for 15 min at 100 V and visualized under UV light after ethidium bromide staining. The bands of specific genes were normalized using GAPDH as s reference.
The cells were lysed in lysis buffer (20 mM Tris, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP-40, 10 mg mL−1 aprotinin, 10 mg mL−1 leupeptin, and 1 mM PMSF) for 60 min and then centrifuged at 12,000 rpm and 4℃ for 15 min. The protein concentrations were determined using the BCA protein assay kit (Thermo scientific, Rockford, IL, USA). The lysate containing 40 μg of protein was subjected to electrophoresis on a sodium dodecyl sulfate-polyacrylamide gel, and the gel was transferred onto a nitrocellulose membrane. The membrane was blocked with 5% non fat dry milk in TBS-T (25 mM Tris-HCl, 137 mM NaCl, 2.65 mM KCl, and 0.05% Tween 20, pH 7.4) for 2 h. The primary antibodies were used at a 1:1,000 dilution. The membranes were incubated with the primary antibodies at 4℃ overnight, washed with TBS-T and then incubated with the secondary antibodies at 1:3,000 dilutions. The signals were developed using an ECL western blotting detection kit and quantified using the Multi Gauge V3.0 software (Fujifilm Life Science, Tokyo, Japan).
All of data are presented as the means ± standard deviation (SD). The mean values were calculated based on data from at least three independent experiments conducted on separate days using freshly prepared reagents. The data were analyzed using the analysis of variance (ANOVA) test of the statistical package for the social sciences (SPSS Inc., Chicago, IL, USA). Significance differences between treatment groups were determined using Duncan’s multiple range tests. The statistical significance of the differences was defined at the p < 0.05 and p < 0.01 levels.
>
Cytotoxicity of the CJE and its inhibitory effect on NO production
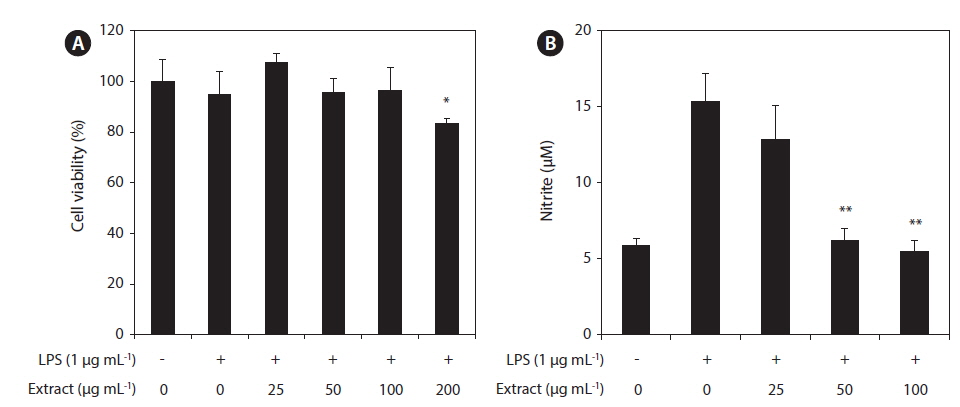
In this study, the cytotoxicity of the CJE in RAW 264.7 macrophages was evaluated using the MTT assay at multiple CJE concentrations (25, 50, 100, and 200 μg mL−1). The CJE did not show cytotoxicity at concentrations up to 100 μg mL−1 compared with untreated cells (Fig. 2A). These lower concentrations were therefore used in the subsequent experiments. To evaluate whether the CJE exquantity erts a potential anti-inflammatory effect in LPS-stimulated RAW 264.7 macrophages, we investigated the inhibitory effect of the CJE on NO production. LPS alone was able to markedly induce higher NO production in these cells than that observed in the untreated cells. However, the CJE significantly inhibited the levels of NO production induced by LPS in a dose-dependent manner (Fig. 2B).
>
Effects of the CJE on LPS-induced iNOS and COX-2 protein and mRNA expressions
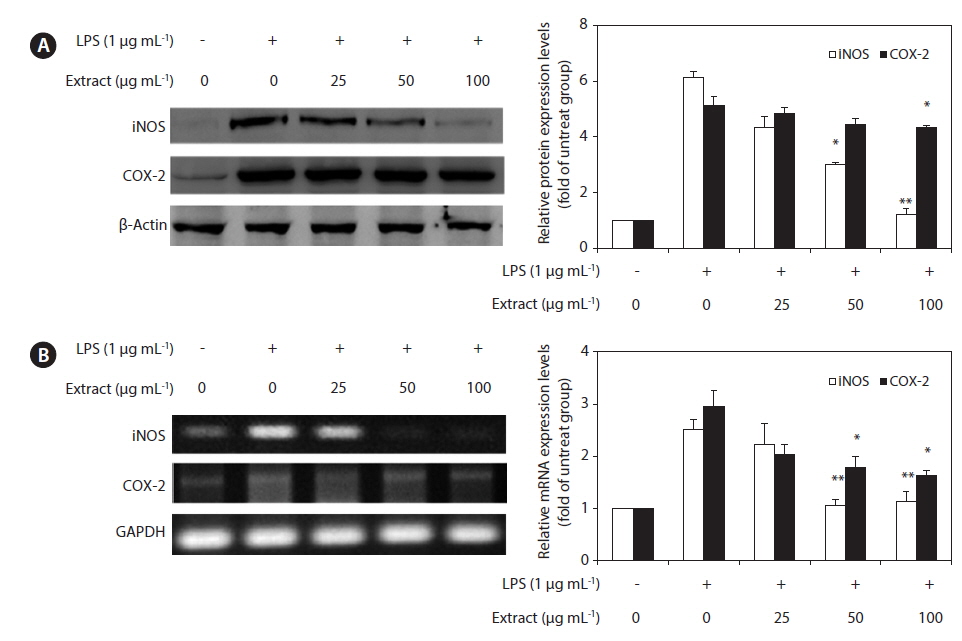
To elucidate the mechanism involved in the inhibition of NO generation by the CJE in LPS-induced RAW 264.7 cells, we further studied the effect of the CJE on iNOS and COX-2 protein and gene expression by western blot and RT-PCR analysis (Fig. 3). In response to LPS, the expression of iNOS was markedly increased, and the CJE significantly inhibited the iNOS and COX-2 protein expression levels in a dose-dependent manner (Fig. 3A). Under the same conditions, the levels of iNOS and COX-2 mRNA expression were correlated with their protein levels (Fig.3B). Similar patterns were observed in the analysis of the effect of the CJE on LPS-induced iNOS and COX-2 protein and mRNA expression.
>
Effects of the CJE on LPS-induced transcription of pro-inflammatory cytokines
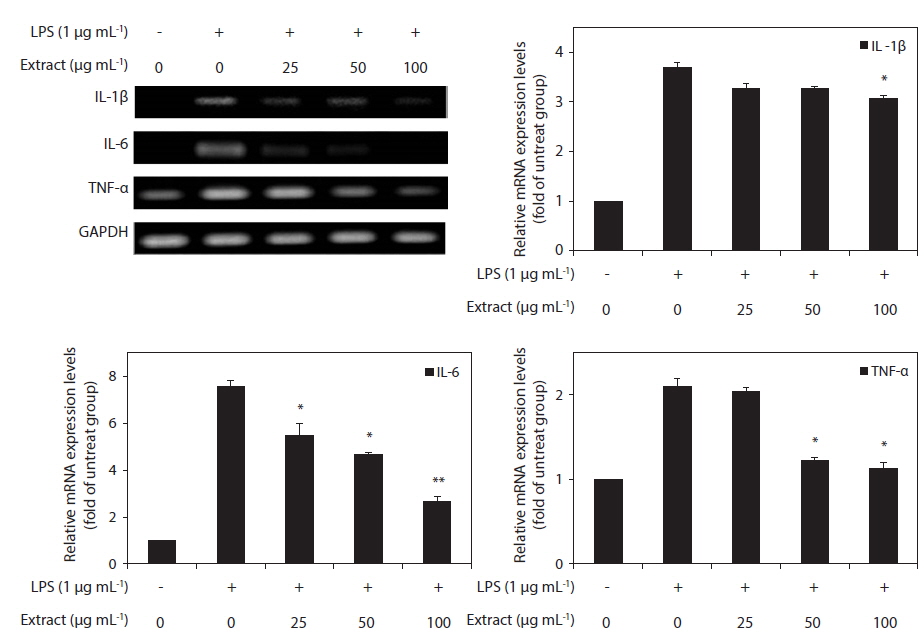
Because IL-1β, IL-6, and TNF-α are pro-inflammatory cytokines that are secreted early and because their levels are elevated in various acute and chronic inflammatory diseases, we determined the effects of the CJE on the mRNA levels of IL-1β, IL-6, and TNF-α in macrophages after treatment with LPS. RT-PCR was performed to determine whether the CJE reduced the expression of those cytokines at the mRNA levels. All of the mRNA levels were increased by treatment with LPS, and these increases were significantly decreased in a concentration-dependent manner by treatment with the CJE (Fig. 4). This finding suggests that the CJE is capable of disrupting key signal transduction pathways activated by LPS in macrophages, subsequently preventing the transcription of pro-inflammatory mediators.
>
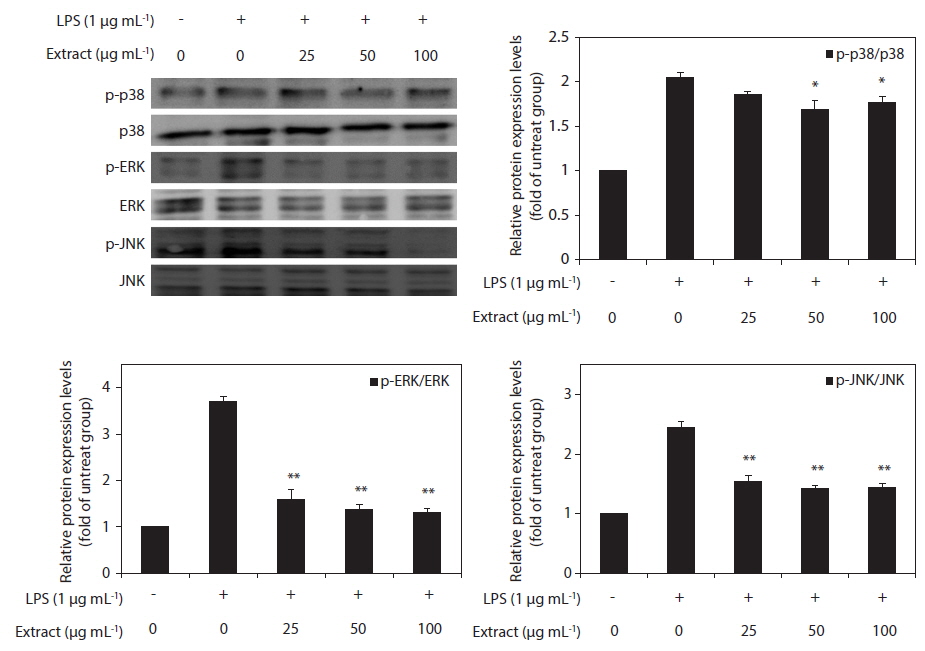
Effect of CJE on LPS-induced MAPKs activation
To elucidate the mechanism of the action of the CJE, we investigated the effect of the CJE on the activation of MAPKs in LPS-activated macrophages by western blot analysis. The levels of phosphorylated ERK, JNK, and p38 MAPK were elevated in the macrophages after treatment with LPS, whereas the levels of total ERK, JNK, and p38 MAPK did not show any significant changes (Fig. 5). However, compared with the cells treated with LPS, the macrophages pretreated with the CJE showed significantly lower LPS-induced activation levels of these proteins (Fig. 5). This result indicates that the phosphorylation of MAPKs was effectively blocked by the CJE in LPS-activated macrophages. Together with the present results, these findings support the conclusion that the CJE, which is obtained from red algae that contains polyphenols, inhibits the inflammation process through inhibiting the expression of pro-inflammatory cytokines in macrophages by down-regulating the LPS-induced activation of MAPK signaling.
Red algae are known as the largest species among algae, present the greates chemical diversity recorded to date in the literature, and supply many useful substances for the treatment diseases that affect living beings. These algae are a source of variable bioactive components, such as polyphenols, alkaloids, and terpenes, and particularly polyphenols, which considered one of the most numerous and widely distributed chemical groups. Based on their structures, red algae are classified into different families with different contents of phenolic acids, phenylpropanoids, flavonoids, and the less-common lignans and stilbenes and have been the object of intense interest due to their various pharmacological activities such as antioxidative, α-glucosidase inhibitory, antimicrobial, aldose reductase inhibitory, antitumor, and anti-inflammatory activities (Wang et al. 2005, Ma et al. 2006, Lee et al. 2007
Inflammation is the first response of the immune system to infection and plays a pivotal role in many diseases (Kim et al. 2010, Heo et al. 2010
In addition, the CJE also significantly reduced the LPS-induced mRNA transcription of TNF-α, IL-1β, and IL-6. According to previous reports, the expression of iNOS is stimulated by pro-inflammatory cytokines, including TNF-α and IL-1β, which play key roles in the induction of inflammation in macrophages (De Nardin 2001, Marcus et al. 2003). TNF-α exhibits its pro-inflammatory activity by regulating several intracellular pathways and can stimulate the production or expression of IL-6 and IL-1β. It elicits a number of physiological effects, including septic shock, inflammation, and cytotoxicity (Aggarwal and Natarajan 1996). IL-6 is a multifunctional cytokine released by LPS-activated monocytes that plays a major role in the immune and inflammatory responses (Ghosh and Karin 2002). Additionally, IL-1β is a key pro-inflammatory cytokine that is involved in various pathological conditions, such as rheumatoid arthritis and fever (Van et al. 2009, Lee et al. 2012). Our results demostrate that the CJE significantly inhibits the transcription of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in macrophages stimulated by LPS, suggesting that the blockade of the iNOS/NO pathway by the CJE may be associated with attenuation of the production of TNF-α, IL-1β, and IL-6.
Mechanical signals are important for maintenance of the normal immune system, and mechanical stress contributes significantly to disease initiation and progression. One of the most extensively investigated transduction mechanims involved in the inflammatory process is the MAPK pathway. Furthermore, MAPKs (ERK, p38, and JNK) have previously been implicated in the signaling pathways relevant to LPS-induced inflammation. The three major MAPKs have been shown to regulate iNOS and COX-2 expression and pro-inflammatory cytokine production in LPS-stimulated macrophages (Kim et al. 2013). It has been suggested that p38 performs a pivotal function in the regulation of iNOS and TNF-α expression (Bhat et al. 1998). Additionally, ERK activation is hypothesized to be involved in LPS-induced macrophage responses, including increases in the production of proinflammatory cytokines and iNOS (Bhat et al. 1998, Ajizian et al. 1999, Kim et al. 2013). Furthermore, JNK plays a significant role in the regulation of the expression of LPS-induced iNOS (Uto et al. 2005). Thus, the suppression of MAPKs activation or function is a key mechanism. In the present study, we examined the effects of the CJE on the activation of these MAPKs and found that the CJE suppressed the phosphorylation of MAPKs. The CJE attenuated LPS-induced phosphorylation of ERK, p38, and JNK. Taken together, these results suggest the potential role of ERK, p38, and JNK in the CJE-induced suppression of NO and pro-inflammatory cytokines in activated macrophages.
In conclusion, the present study demonstrates that the CJE is a potent inhibitor of NO, TNF-α, IL-1β, and IL-6 production at the transcriptional level in LPS-stimulated RAW 264.7 cells. The mechanism underlying the inhibition of NO production appears to involve the down-regulation of iNOS and COX-2 protein and mRNA expression, which may be associated with the attenuation of TNF-α, IL-1β, and IL-6 production. Furthermore, the levels of phosphorylated MAPKs were significantly decreased due to pretreatment with the CJE in LPS-stimulated macrophage cells. Taken together, these results indicate that the CJE exerts its anti-inflammatory actions through a blockade of the MAPK pathways in RAW 264.7 macrophages.