The marine red alga Pyropia J. Agardh, segregated from the previous larger genus Porphyra C. Agardh, is an important marine crop with a market currently worth over US $2 billion per year (Blouin et al. 2011), and growing rapidly. Similar to any other agricultural products, Pyropia cultivation beds are plagued by many diseases (Gachon et al. 2010). Among these, red-rot and Olpidiopsis diseases, both oomycete pathogens, are most common, and can reduce outputs by an estimated 20% in some areas (Kawamura et al. 2005, Klochkova et al. 2012). A new Pyropia disease is reported almost every year as cultivation areas are rapidly expanding (Ding and Ma 2005, Guan et al. 2013), and the impact of disease is expected to increase with environmental changes, such as global warming. Although the infected crop is still harvested and used, its yield and quality are seriously lowered and the Pyropia products are less lustrous, uneven and discolored (Klochkova et al. 2012). As some of these diseases may become chronic, they have the potential to cause serious economic damages to sea farmers.
Little is known regarding the mechanism of infection in Pyropia and few pathogens have been isolated for laboratory culture (Gachon et al. 2010). Red-rot disease was first reported by Arasaki (1947) and best studied, yet it took three decades to isolate and name its causative agent, the oomycete Pythium porphyrae Takahashi et Sasaki (Takahashi et al. 1977). Although the ecological nature of epidemics and the physiological characteristics of this pathogen have been investigated intensively, there are very few cellular and molecular studies on the mechanisms of infection (Uppalapati and Fujita 2000a, 2000b, Park et al. 2001a, 2001b, 2006, Uppalapati et al. 2001, Addepalli et al. 2002, Hwang et al. 2009). Other pathogens are more poorly known; the pathogen causing “chytrid blight” of Pyropia in Japan was recently isolated and named Olpidiopsis porphyrae Sekimoto, Yokoo, Kawamura et Honda (Sekimoto et al. 2008). As the disease-causing agent was not a chytrid, the name of the disease was changed to Olpidiopsis disease. The causative agent of green-spot disease is still unknown. “Diatom felt” (i.e., large concentration of epiphytic diatoms) is produced by many different species of diatoms, but the symptoms and the extent of the damage to Pyropia have not been evaluated.
Acid wash is the most common treatment for sea farmers to eliminate epiphytes and pathogens from the cultivated Pyropia. As Pyropia is more tolerant to acidic solutions than other epiphytic organisms this treatment is thought to be quite useful. Experimental data showed that Pyropia blades should be immersed in the acidic solution for more than 5 min to prevent the spread of redrot disease (Sakaguchi et al. 2001). A boat equipped with a large bathtub passes underneath the cultivation net and automatically immerses Pyropia into the acidic solution, while the boat moves forward slowly. Because the boat must cover large areas, the immersing time is usually less than 30 s. The effect of acid wash on some pathogens, such as Olpidiopsis spp. and bacteria, has never been evaluated.
In this study, we summarize the epidemiology of currently recognized Pyropia diseases in Korean sea farms. We present data on the disease incidence rates in two major sea farms that produce over 80% of the Pyropia crop in Korea, and discuss the economic losses caused by each disease, estimated using the account data provided by a sea farming company.
The oomycete Pythium porphyrae was isolated from infected blades of Pyropia yezoensis cultivated in Jindo Island (34°46′ N, 126°36′ E, Southern Sea), Korea in December 2010 and was designated as strain KNU-pyp2. Clonal strain was maintained on agar plates made with Arasaki B medium (Arasaki et al. 1968) at 20℃ with 12 : 12 h L : D cycle and 15 µmol m−2s−1 light intensity. The mycelium was always sterile on the agar plates. To obtain zoospores from P. porphyrae that were used in Pyropia spp. infection experiments, pieces of agar containing mycelium were inoculated into liquid Arasaki B medium and grown for 5-7 days.
Olpidiopsis sp. infected blades of Pyropia spp. were collected from a commercial plantation in Seocheon (36°12′ N, 126°50′ E, Western Sea), Korea on 3 February 2010. Isolated Olpidiopsis strain from Pyropia was designated as strain KNU-M-omc2. Host plants, Pyropia spp., were kept in MGM medium (Klochkova et al. 2012) with constant aeration at 10℃ with 16 : 8 h L : D cycle and 30 µmol m−2s−1 light intensity. Cultures of Olpidiopsis sp. were maintained for over 4 years by transferring a piece of infected blade to a solution containing healthy Pyropia blades every week.
Diatoms from “diatom felt” were isolated from a commercial plantation in Seocheon (36°12′ N, 126°50′ E, Western Sea) and cultured separately in IMR medium (Klochkova et al. 2006) at 10℃ with 16 : 8 h L : D cycle and 30 µmol m−2s−1 light intensity.
Pyropia blades are harvested and sold every week at designated local auction sites during the cultivation period (from early November to late April) by a designated fishery cooperative association. About 80% of Korean Pyropia product is sold in Haenam-Jindo and Seocheon auctions. Samples of Pyropia product were collected from two auctions and transferred to the laboratory every two weeks. Each time, the probe consisted of several kg of raw material. The comparative incidence rate could not be estimated from a whole blade, because almost all of the Pyropia blades were suffering from disease to some extent. Thus, because the cultivated Pyropia blades are flattened, they were cut into 1-cm2-sized pieces with a razor blade and put on a plotting paper, and the incidence rate of each disease was calculated from the following equation:
Infected area of each disease / Total area of a blade × 100
About 6% of Korean Pyropia is produced in the Seocheon area and auctioned by a company, Seocheon Fisheries Cooperative Association (Seobu-Suhyop, Seocheon, Korea). The company allowed us access to the account data for every sales week for the last three years (2011-2014 seasons). The economic loss caused by each disease was estimated by combining the outbreak data of a disease with the weekly sales data (i.e., total income, total production, prices per 1 kg) of Pyropia product at the auction place.
Light micrographs were taken with Olympus DP73 computer controlled CCD camera affixed to an Olympus BX50 microscope (Olympus, Tokyo, Japan). For transmission electron microscope (TEM), Pyropia blades brought from Haenam-Jindo and Seocheon sea farms were fixed on the same day in 2.5% glutaraldehyde in phosphate buffered saline (PBS) buffer with an adjusted sodium chloride concentration at 4℃ for 2 h. Samples were then rinsed with the same buffer and post-fixed with 1% osmium tetroxide at 4℃ for 2 h. Thereafter, the tissues were rinsed out with PBS buffer and were dehydrated in a graded ethanol series with 10% increments (each step was 20 min), embedded in Spurr’s epoxy resin and polymerized overnight at 72℃ oven (Polysciences Inc., Warrington, PA, USA). Thin sections were stained with 4% uranyl acetate for 30 min and lead citrate for 10 min, and were viewed and photographed on a Hitachi H-300 TEM (Hitachi, Tokyo, Japan).
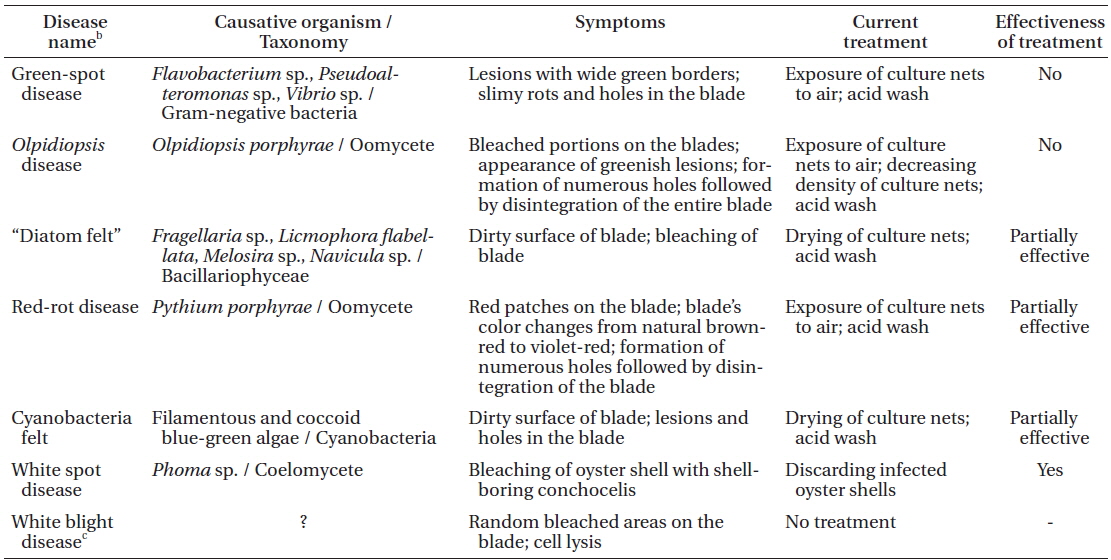
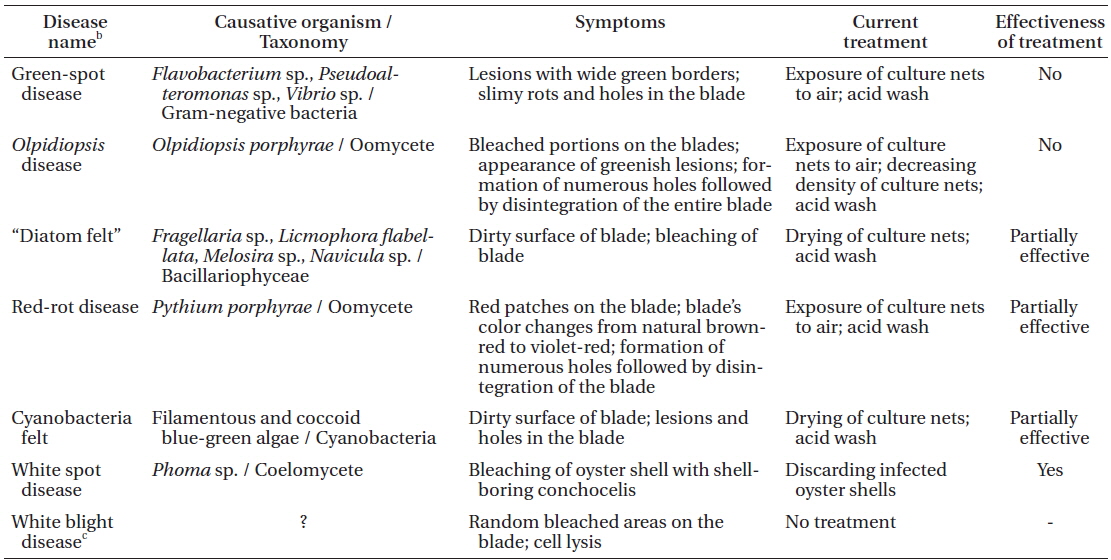
The symptoms of some Pyropia diseases have not been described in detail to date. Moreover, some diseases have several different names, which can be misleading. Local names, disease case reporting and detailed morphological characteristics of each disease in Korean Pyropia sea farms are summarized below. Commonly occurring Pyropia diseases in Korean sea farms and current control measures are summarized in Table 1.
Other names used in references. Bulgeun-gaet-byong (Korean name: 붉은갯병); Gochugaru-byong (Korean name: 고춧가루병); Balgang-byong (Korean name: 빨간병); Akagusare-byo (Japanese name: 赤腐れ病); Chìfǔbìng (Chinese name: 赤腐病); laver brown rot; rot disease; red wasting disease; wasting disease.
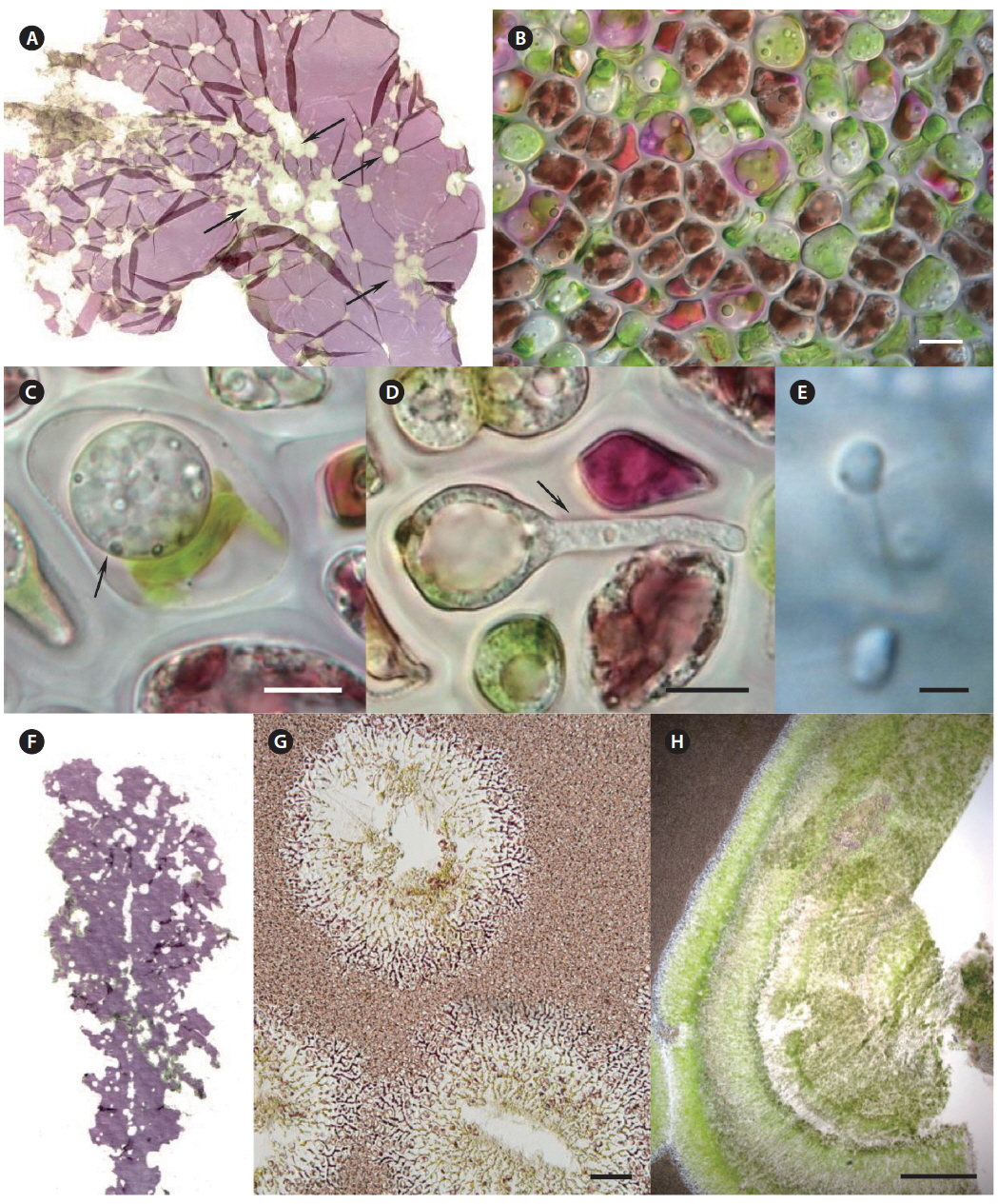
Morphological characteristics. Red-rot disease of Pyropia (Fig. 1A & B) is recognizable by the appearance of distinct, small, red patches on the blades in areas where the zoospores of Pythium porphyrae germinated (Fig. 1D-F), and where the mycelium grew through the host cells and already killed them (Fig. 1C). Dead host cells change color from natural brown-red to violet-red before they turn green and degenerate completely. The infection spreads quickly to other areas on the blade, and dead host tissue deteriorates forming numerous small holes. The holes merge into bigger holes, ultimately disintegrating the entire blade.
Disease case reporting. Red-rot disease was reported as one of the major constraints in the profitable cultivation of Pyropia in China, Korea, and Japan. “Akagusarebyo” or rot disease on Pyropia (Porphyra) was first diagnosed in Japan (Arasaki 1947). The causative agents of red-rot disease in Pyropia were reported to be the oomycetes Pythium marinum Sparrow and P. porphyrae (Fuller et al. 1966, Sasaki and Sato 1969, Kazama and Fuller 1970, Sasaki and Sakurai 1972, Sakurai et al. 1974, Fujita and Zenitani 1976, Takahashi et al. 1977, Aleem 1980, Tsukidate 1983, Kerwin et al. 1992, Amano et al. 1996, Shin 2003a, 2003b). Molecular phylogenetic analysis of 102 isolates of Pythium spp. suggested that P. marinum was phylogenetically distinct from P. porphyrae (Lé Vesque and De Cock 2004); however, it must be noted that P. marinum isolate (GenBank No. AY598689) used by Lé Vesque and De Cock originated from a soil sample collected from the UK, rather than a marine site. That soil strain no longer formed sexual structures to verify its morphological identity, and its identity was doubted by Lé Vesque and De Cock (2004) themselves. Moreover, P. marinum was originally described from Ceramium rubrum C. Agardh in Denmark, and there is no evidence indicating that it caused death in this red alga (Sparrow 1934). No molecular data, other than phylogenetic markers, are currently available for P. porphyrae and P. marinum. It is our understanding that there is no P. marinum parasite on Pyropia. In Korea, redrot disease was shown to be caused only by P. porphyrae (Park et al. 2001a, 2001c, 2006).
Other names used in references. Hosang-kyun-byong (Korean name: 호상균병); Tsubozyo-kin-byo (Japanese name); Gojyo-kin-byo (Japanese name: こじょうきん病); Húzhuàngjūnbìng (Chinese name: 壶状菌柄); chytrid blight; chytridiosis.
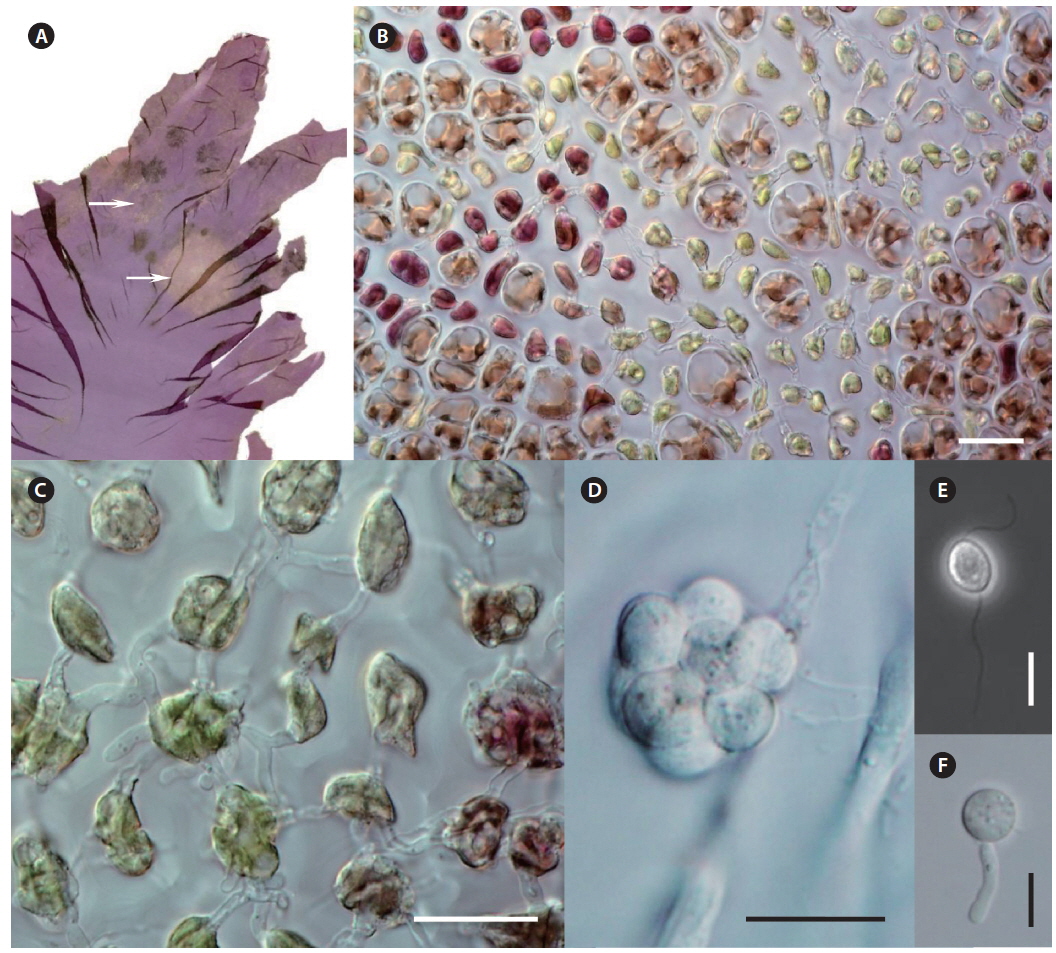
Morphological characteristics. Olpidiopsis is an obligate endoparasite, infecting many different species of Pyropia (Sekimoto et al. 2008, 2009). We recorded Olpidiopsis spp. disease on Pyropia from Korean sea farms (Klochkova et al. 2012). It is recognizable by the appearance of distinct bleached portions on blades at the initial stages. Upon progression of the infection, the host tissue breaks down and greenish lesions appear (Fig. 2A & B). The infection process starts when encysted zoospores of Olpidiopsis attach to the surface of Pyropia and produce thin germ tubes that penetrate the cell walls of the host. After entering the host cell, Olpidiopsis forms spherical multinucleate thalli (Fig. 2C), which develop into fully-grown zoosporangia within the next 2 days (Fig. 2D). Zoospores (Fig. 2E) escape the zoosporangium through a discharge tube, which forms at the final developmental stage. After zoospores escape, the lysed cell wall matrix of Pyropia remains containing the degenerated cytoplasm.
Disease case reporting. Olpidiopsis disease has been reported in China, Korea, and Japan. “Tsubozyo-kin-byo,” or chytrid blight disease, on Pyropia (Porphyra) was first reported by Arasaki (1960) from Japan. Migita (1969) proposed that it should be called Olpidiopsis disease, because the causative agent is not a chytrid. In Korea, Olpidiopsis spp. on Pyropia (Porphyra) has been recorded since 1986; however, the pathogen has not been commented on (Cho and Chang 1986).
To date, molecular phylogenetic analysis has been carried out on only one species of Pyropia-infecting Olpidiopsis, O. porphyrae from Japan (Sekimoto et al. 2008).
Green-spot disease in Pyropia (Porphyra) was reported over twenty years ago in Korea. However, detailed illustrations depicting its specific symptoms and infecting agents have not been summarized to date.
Other names used in references. Nokban-byong (Korean name: 녹반병, English name: green-spot disease); Anaaki disease (Japanese name: 穴あき病, English name: ulcer disease); “Perforating Disease” in Nori; Lǜbānbìng (Chinese name: 绿斑病); blight; pore disease.
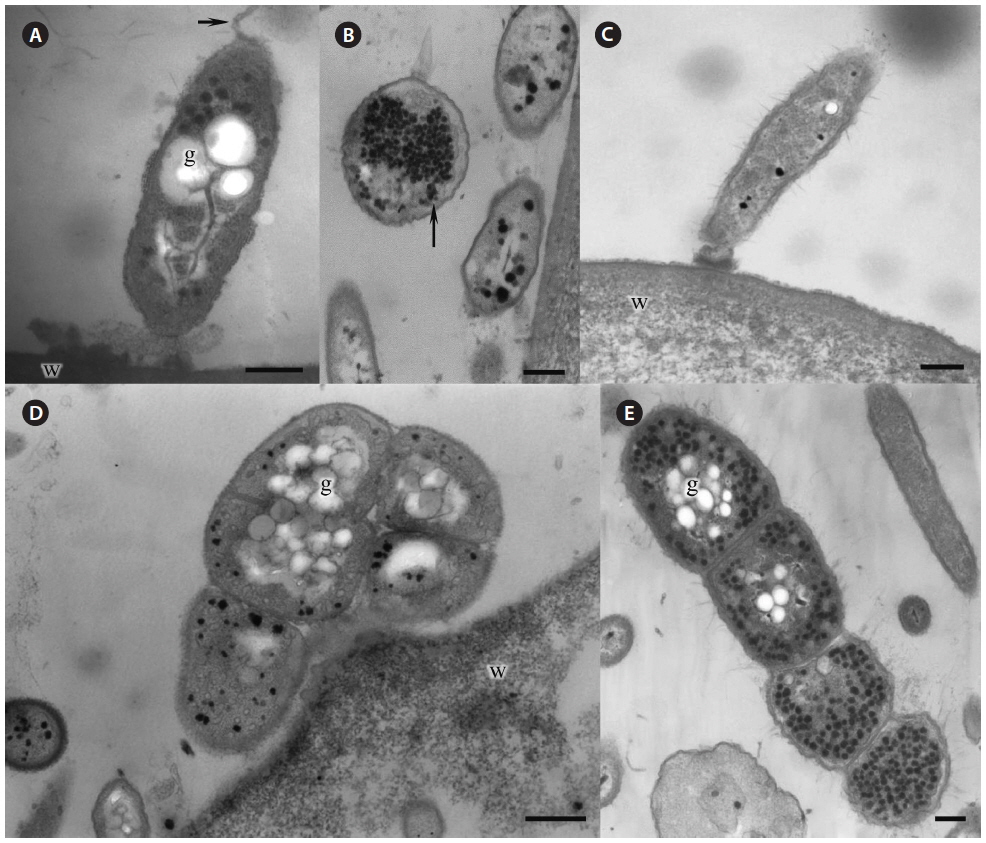
Morphological characteristics. Green-spot disease in Pyropia is recognizable by the appearance of relatively small, distinct lesions on the blade with defined, wide, green borders (Fig. 2F-H). The lesions can occur anywhere on the blade, and often show severe bacterial contamination. As lesions grow and coalesce, slimy rots occur when host tissue breaks down. Thereafter, holes are created in the blades. In our samples, the surface of Pyropia near the lesion border was covered with bacteria at densities of approximately 60-110 cells per 50 µm surface length (counted in TEM sections). Perpendicularly attached bacteria secreted visible layer of mucilage at the point of attachment to the host surfaces (Fig. 3A & C).
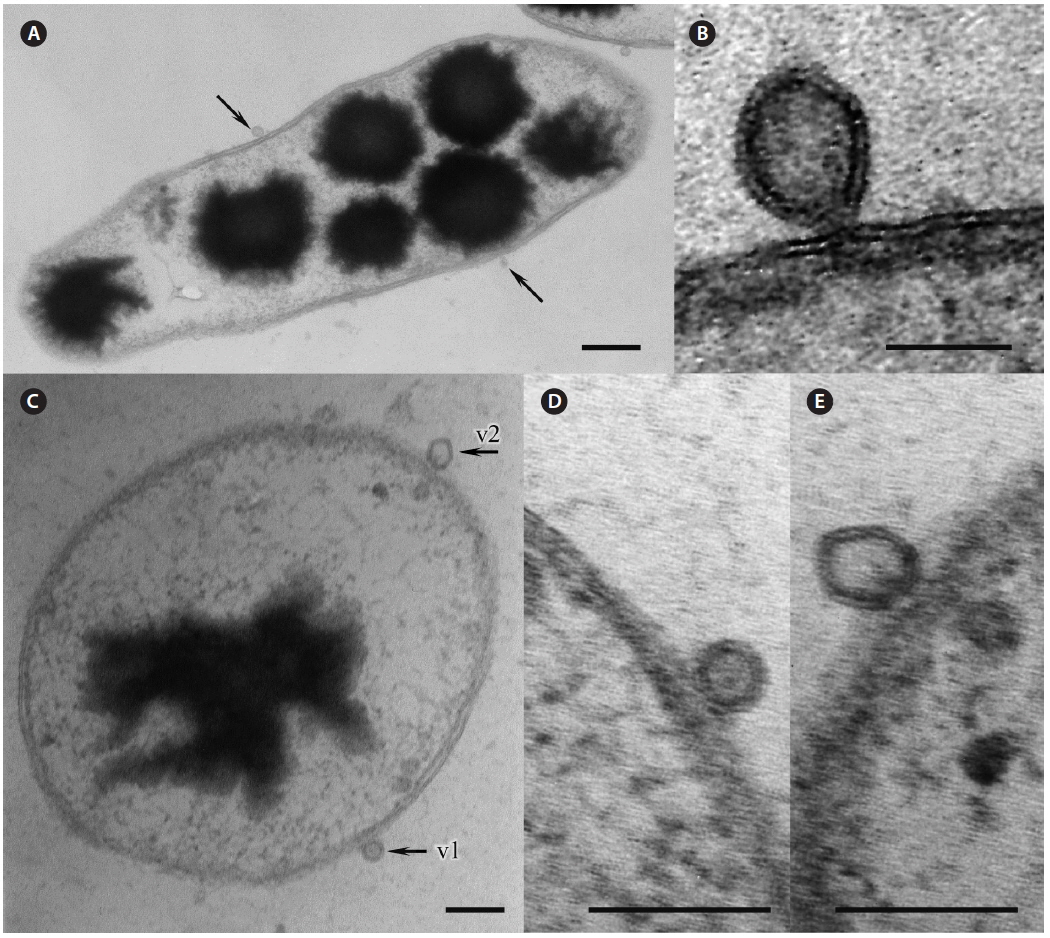
Many bacteria were undergoing binary fission and had attached to Pyropia as long chains. About 82.5% of bacteria were packed with numerous black polyhedral inclusion bodies (enterosomes or phages). In many cells, the inclusion bodies were so numerous that they filled almost the entire cell (Fig. 3B). Many bacteria attached to Pyropia had large reserve granules (Fig. 3A). In the debris from disintegrated Pyropia tissue, numerous bacteria with membrane vesicles resembling outer membrane vesicles (OMVs) were observed (Fig. 4A & C). The vesicles were between 50 and 200 nm in diameter and surrounded by a membrane bilayer (Fig. 4B, D & E).
Most Gram-negative bacteria have OMVs that shed from their outer membrane, and extracellular secretion of products is the major mechanism by which these pathogens communicate with and intoxicate host cells (Kuehn and Kesty 2005). Further, macromolecule-degrading enzymes in association with OMVs can promote nutrient acquisition (Shevchuk et al. 2011). Surface appendages, such as fimbriae and flagella, are required for bacterial motility and pathogenicity (Liles et al. 1998, Stone and Abu Kwaik 1998, Heuner and Steinert 2003). Reserve granules (i.e., glycogen) are produced in bacteria when the creation of flagella, fimbriae, and components of the capsule is repressed and bacteria lose their capacity to move and adhere to surfaces. Altogether, this implies that the bacteria found on the surface of Pyropia and in the debris from disintegrated Pyropia tissue are pathogens that feed off its host.
Disease case reporting. To date, several different species of bacteria are thought to be potential causative agents of green-spot disease in Pyropia. Aerobic Gramnegative bacteria, such as Pseudomonas and Vibrio, were found to be associated with green-spot disease in P. yezoensis in Japan (Fujita 1990). Further, Anaaki disease (i.e., green-spot disease), which severely damaged P. yezoensis in Japan, was reported to be caused by Flavobacterium sp. LAD-1 (Sunairi et al. 1995). Marine agarolytic Pseudomonas sp. was isolated in Korea from Porphyra dentata (Pyropia dentata) showing symptoms of green-spot disease (Park et al. 2001c). The isolated Pseudomonas strain from P. dentata had carboxymethyl cellulase, xylanase, protease, and agarase activities, and was able to macerate algal tissue 1 week after inoculation at pH 7 and 30℃ (Park et al. 2001c). However, this infection process was different from the progress of green-spot disease on Pyropia blades that we observed: first, the infection occurs when seawater temperature is below 10℃, and the green-spot disease can degenerate a whole blade within a day or two; second, the blade does not undergo typical maceration process; rather, infected Pyropia cells burst successively from the initial infection site, forming a row of dead cells.
Other names used in references. Sīzhuàngxìjūnbìng (Chinese name: 丝狀细菌病); filamentous bacteria disease; filamentous bacterial felt disease.
Morphological characteristics. Very often, green-spot disease and cyanobacteria felt occur simultaneously on Pyropia; therefore, their symptoms coincide. The cuticle layer of Pyropia loosens and degenerates when abundant bacteria and cyanobacteria attach. A distinct long “bristle” is visible on the blade’s surface, but is thickest at the very edge of the lesion, facing the exterior of the lesion. Both individual cells and large colonies of coccoid cyanobacteria secrete abundant mucilage that was observable with TEM. Further tubercles can develop in the places where they adhere to the host surfaces. In our samples, cyanobacteria cells contained numerous reserve granules and cyanophycin granules (Fig. 3D & E).
Disease case reporting. Filamentous bacterial felt disease has been reported in Korea by Lee et al. (2012). During our own sampling from 2012 to 2014, we isolated coccoid and filamentous cyanobacteria from Pyropia in sea farms and natural populations in Korea (Fig. 3D & E). Because the cyanobacteria had diverse morphologies, the disease name should not include the word “filamentous.”
The surfaces of macroalgae are typically covered by bacteria at densities of approximately 107 bacteria cm−2 (Armstrong et al. 2000). Further, bacterial colonization is often host-specific. Pseudoalteromonas spp., Vibrio spp., and blue-green algae occur in normal marine and brackish waters, thus avoiding contact with Pyropia cultivation beds is not possible. The flavobacteria species that we found are known as freshwater pathogens and soil saprophytes. Thus, they might have been brought to the sea farms with sewage or other freshwater stocks. Rapid growth of these organisms and subsequent outbreak of infectious diseases can only be regulated by maintaining satisfactory seawater quality near the sea farms.
Other names used in local farms. Dook or Beol (Korean name: 뚝, 뻘, mainly for Navicula), Doda (Korean name: 도다, mainly for Licmophora).
Morphological characteristics. “Diatom felt” on Pyropia appears as distinct brown fringe all over the surface of the blade. When touched with fingers, detached diatoms are seen, thus this disease can be easily recognized by non-professionals. Growth of Pyropia can be seriously affected by epiphytic diatoms, as they shade light, compete for nutrients, and cause bleaching of macroalgal thalli. Infected Pyropia has a distinct, unpleasant, earthy odor.
Although this disease does not cause serious production loss, farmers are most concerned as it is directly linked to the price of raw material. Attached diatom cells cannot be washed away completely and the remaining diatoms clog the pores of sponges, which are used during automatic laver sheet pressing to adsorb residual water during processing. This results in low product quality and increased production costs, due to frequent sponge replacement. Further, diatom oil can stick to the blades of cutting devices, thereby causing malfunction of processing machine.
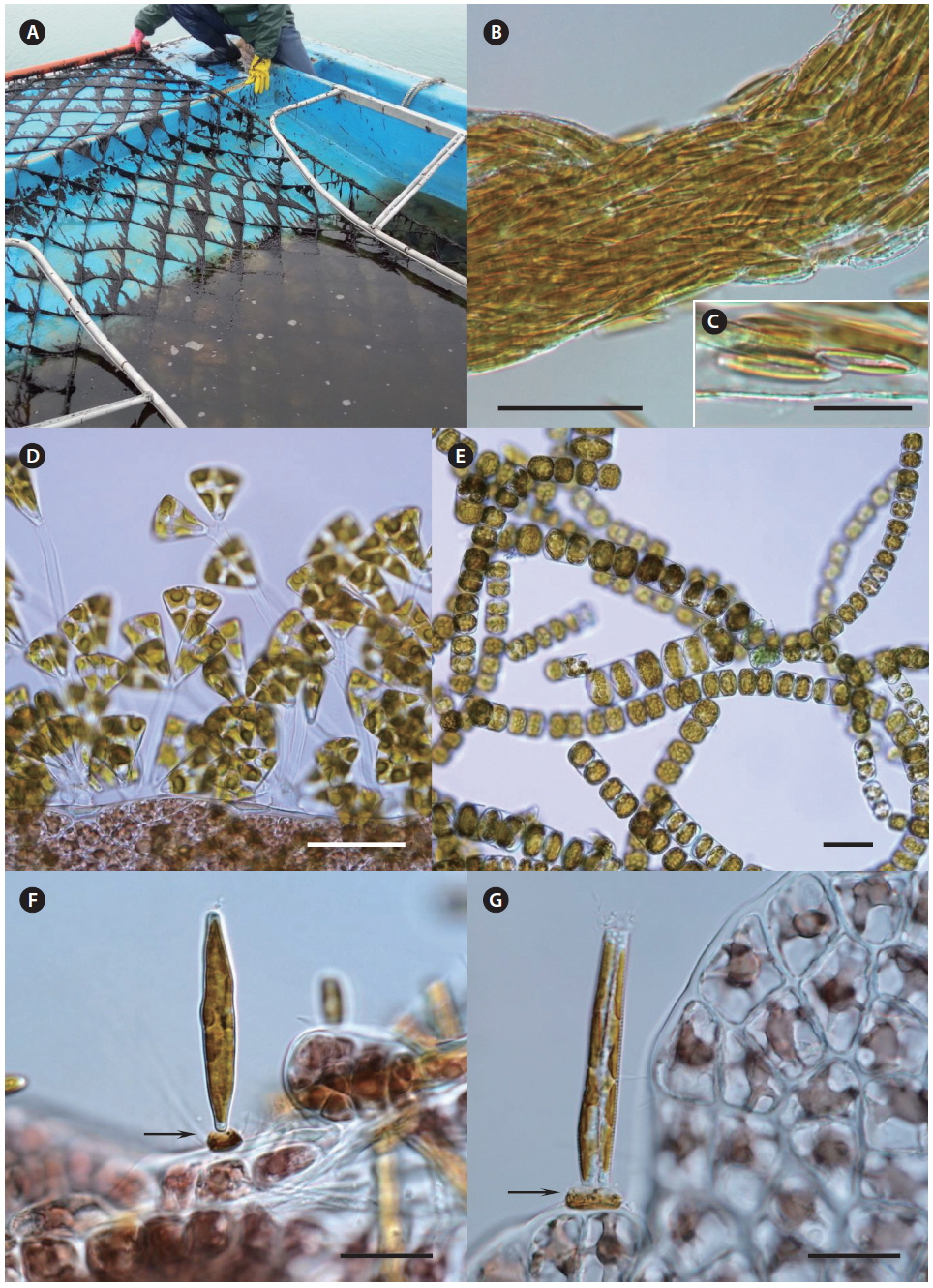
Disease case reporting. Pennate diatoms Navicula spp. and Licmophora spp. are the most common agents causing “diatom felt” in Pyropia sea farms in Korea. Their attachment was highest in November 2008 (Lee et al. 2012). During our own sampling, we found severe contamination of Pyropia sea farm with the pennate diatoms Fragellaria spp., Licmophora flabellata, tube dwelling Navicula spp. and the centric diatom Melosira moniliformis (Fig. 5).
As stated in a recent review on Pyropia crops (Blouin et al. 2011), fouling organisms (e.g., macroalgal spores, diatoms, and invertebrate larvae) are killed by desiccation when Pyropia nets are raised out of the sea by farmers, several hours a day, several times a week, especially early in the growing season, whereas Pyropia survives. Our field work in Pyropia sea farms and laboratory-controlled experiments do not support this statement. The outbreak of each disease is seasonal, so raising the nets out of the sea early in the growing season (i.e., late October-early November) would not inhibit or eliminate “diatom felt” and other disease-causing agents if the outbreak happens months later. Moreover, the inverted and floating nets that are currently used in the sea farms are not raised fully out of the sea and complete desiccation does not occur. Finally, mucilage-embedded organisms, including diatoms, are notoriously durable and cannot be killed through short-term desiccation. For example, silica gel-preserved dry marine planktonic diatoms Navicula spp. can remain viable for up to three months (T. A. K. personal data). Other most prevalent Pyropia disease-causing agents, such as oomycetes, are well-protected within host cells and do not die due to desiccation unless their host dies with them. Bacteria remain viable in the dry and frozen states for many days. To date, no treatment exists for Pyropia plantations infected with “diatom felt” and other parasites, with the exception of a controversial acid wash of the nets.
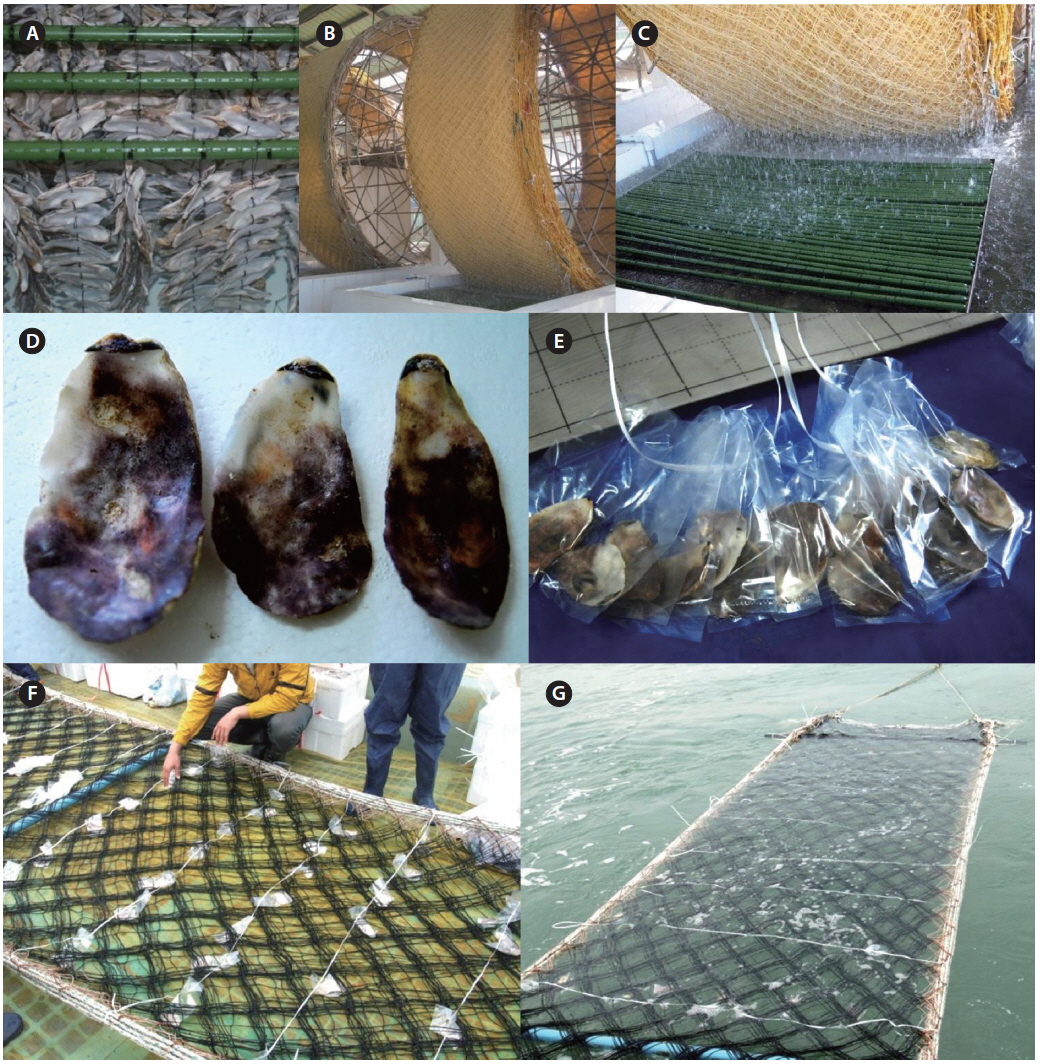
The seeding of Pyropia farms starts in early October in Korea. For commercially operated ground seeding, the spores are released from conchocelis filaments grown on oyster shells during the summer (Fig. 6A) and gathered in large tanks from a seeding company (Fig. 6B). The cultivation nets are immersed in the tanks and rotate mechanically similar to a water mill for several days until enough spores have attached (Fig. 6C). To maintain the crop quality, this ground seeding is preferable to natural seeding, although its cost is more than two times higher. Natural seeding is achieved directly in the sea farms using several different methods, depending on a farm. For example, numerous polyvinyl bags containing crushed or whole oyster shells with conchocelis filaments (Fig. 6D & E) are hung under the cultivation nets (Fig. 6F), and the nets are immersed in seawater for 2-3 weeks until enough spores attach to the nets (Fig. 6G). When the seeding is finished, the cultivation nets are moved to the ground again and stored at −20℃ for several weeks until the temperature of the seawater drops below 15℃. This freezing period is necessary to prevent an early outbreak of diseases and run the farm on schedule.
The seeding of a Pyropia farm can run behind schedule due to an outbreak of white spot disease, which is caused by a coelomycetous soil fungus from the genus Phoma, which can infect the shell-boring conchocelis stage of Pyropia yezoensis (Guan et al. 2013). However, this disease was not reported among 11 Pyropia-seeding companies in Korea from 2011 to 2014, and the economic damage caused by this disease seems minimal even when it occurs (personal communication). The oyster shells that contract this disease are easily spotted and discarded.
In Korea, Pyropia diseases began to occur soon after the cultivation net was established in the sea (October); however, the first outbreak of disease usually occurred in late November, one or two weeks after the first harvest. When the same harvesting ship passed underneath the cultivation nets in the sea farm to cut the Pyropia blades, diseases were likely transferred. After the harvest, wounded blades were infected more easily with diseases, such as cyanobacteria felt and green-spot disease.
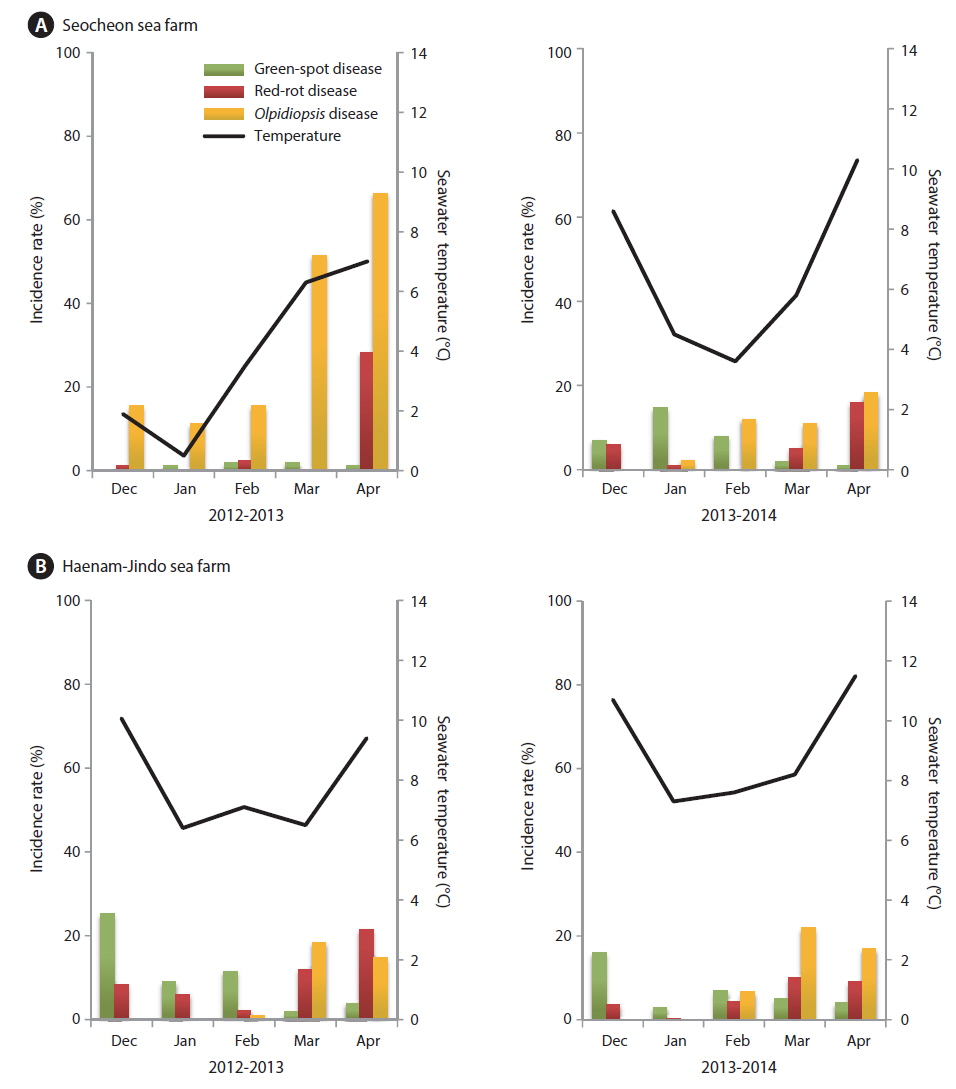
The incidence rates of diseases in our study (Fig. 7) were different from those published by Lee et al. (2012). Although red-rot disease caused by P. porphyrae was still a serious problem in the surveyed Pyropia farms, it was not the most common disease. During the 2012-2013 season, an outbreak of Olpidiopsis spp. disease occurred in the Seocheon sea farms, and lasted for the whole season (Fig. 7A). Because of the Olpidiopsis outbreak, the first harvest of Pyropia was delayed for 1-2 weeks, and the sea farms closed 1 month earlier than usual (Fig. 8). At the Haenam-Jindo sea farms, an outbreak of green-spot disease occurred together with red-rot disease during the 2012-2013 season (Fig. 7B), and many sea farms could not even start cultivation until late December. In the following 2013-2014 season, an outbreak of green-spot disease caused great economic damage in this region. Redrot disease was most likely a chronic disease in both sea farms; however, when it occurred with other diseases, the impact of the damage was more devastating.
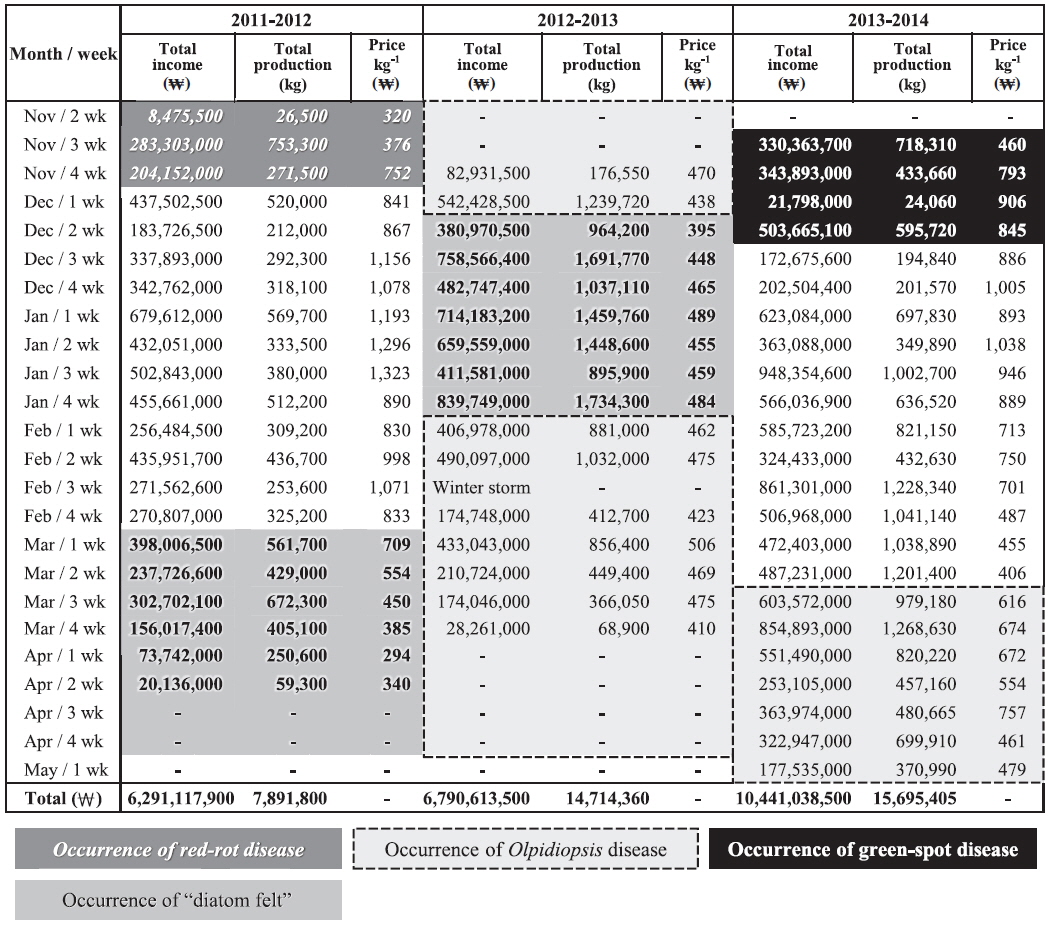
Pyropia blades are harvested every week and directly sold at the local auction by a designated fisheries cooperative association. We analyzed the weekly sales data of the Seocheon fisheries cooperative association (Seobu-Suhyop) from 2011 to 2014 in connection with the occurrence of each disease (Fig. 8). During the 2012-2013 season, Seocheon sea farms suffered from an outbreak of Olpidiopsis spp. disease (Fig. 7A) and had to close down one month earlier than usual. This cost the company approximately US $1.6 million, which was approximately equivalent to 24.5% of total sales for the 2012-2013 season in Seocheon (Fig. 8).
“Diatom felt” has often been regarded as a minor nuisance in Pyropia farming because it does not cause significant production loss. However, our data showed that it may cause serious economic loss to sea farmers by lowering auction price. “Diatom felt” plagued Seocheon sea farms during the 2011-2013 seasons, and the damage was devastating. Although there was little production loss during this period, the price per weight dropped to 1/3 of the other seasons evaluated (Fig. 8). As the Pyropia products with “diatom felt” were uneven in shape and less tasty, the auctioneer sometimes stopped the auction when there were too many products with “diatom felt.” The buyers at the auction also explained that Pyropia blades with “diatom felt” caused a malfunction of the laver-processing machine, and that controlling “diatom felt” is the main reason for acid wash.
An outbreak of green-spot disease occurred in Seocheon and Haenam-Jindo sea farms during early December 2013 (Fig. 7). Although the infected crop was still harvested and used, crop yield and quality were seriously lowered. The disease resulted in an expense of approximately US $1.1 million, or 10.7% of the total sales for the 2013-2014 season in Seocheon (Fig. 8). During the 2012-2013 season, a severe outbreak of this disease occurred in sea farms in Haenam-Jindo, and nearly 1/3 of the sea farms shut down for almost an entire season. After the auction, Pyropia blades are transferred to a processing company and dried to a final product, Nori sheets (Korean name: 김, Gim). The price of the dried product is 5-10 times higher than the auction price of raw Pyropia blades. Therefore, the actual economic damage to local fisheries caused by Pyropia diseases may be much bigger than our estimation.
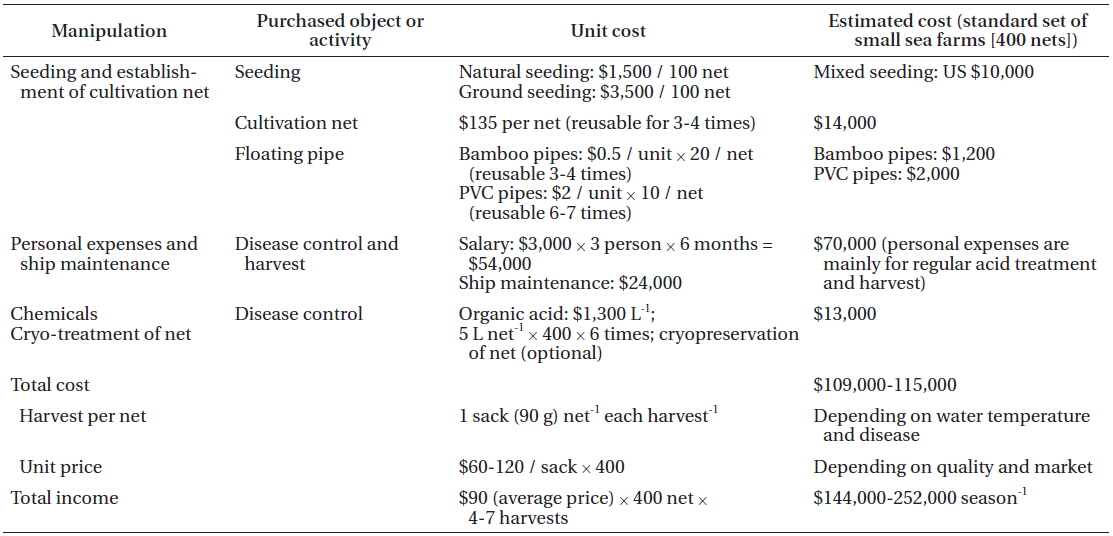
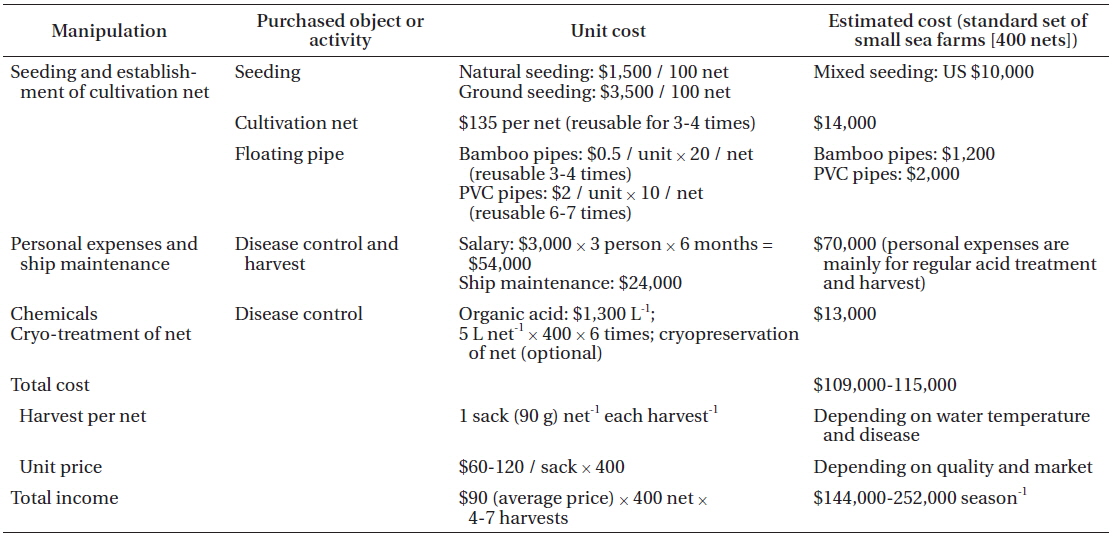
To analyze the cost of disease control in Pyropia farming, we worked with a family who has run a small-sized (30 ha) sea farm in Seocheon for two seasons. Pyropia farming consists of four main activities: seeding, establishment of cultivation nets, disease control, and harvesting (Table 2). The cost of disease control was higher than any other activity. Acid wash is used by farmers for almost all Pyropia diseases; however, it is primarily used to control epiphytes, including diatoms (Table 1). Although sea farmers prefer to use diluted hydrochloric acid rather than the organic acid mixture because of the price (commercial grade hydrochloric acid 1 L = 500 won vs. citric acid 1 kg = 52,000 won), its use is banned by environment law. A commonly used reagent for the acid wash is a mixture of several different organic acids such as acetic, formic, citric, oxalic, and uric acid, with 10% hydrochloric acid.
The cost of the acid for disease control was about 12% of the total cost. Considering that people are hired in Pyropia sea farms mainly for the acid treatment and harvesting process, the cost for disease control occupies close to half of the total cost.
Disease control is a key process for successful Pyropia farming. The impact of disease is increasing every year, because of the recent development of intensive and dense mariculture practices and global environment change. Economic loss caused by Pyropia disease and the cost of current control measures were evaluated from a case study in Seocheon, Korea. Results showed that the cost of disease control occupies almost half of the total farming cost, but Pyropia sea farms still suffer from many diseases even with these measures. These disease control measures seem to have little impact. Seocheon Seobu-Suhyop, which occupies about 5% of total Pyropia production in Korea, lost approximately US $1.6 million during the 2012-2013 season, because of Olpidiopsis spp. disease. Therefore, total economic loss caused by Pyropia diseases may be well over US $10 million per year in Korea. There are very few studies on the infection mechanism of Pyropia diseases and the causing agents of some diseases, i.e., green-spot disease, have not been identified. Epiphytic diatoms, which do not directly damage Pyropia cells, may cause more serious economic loss than any other disease. It is necessary to develop a comprehensive manual including proper chemical treatments to protect Pyropia farms from disease outbreaks.