Dinoflagellates are often abundant and ubiquitous protists in marine environments (Gómez 2012, Jeong et al. 2013, Kang et al. 2013, Yoo et al. 2013, Moestrup et al. 2014). They play diverse roles in marine ecosystems as producers, predators, prey, competitors, parasites, and symbiotic partners (Hansen 1991, Jeong 1999, Stoecker 1999, Kim et al. 2013, Park et al. 2013, Seong and Jeong 2013, Lim et al. 2014, Lee et al. in press). The ecological niche of a dinoflagellate species is usually different from that of the other dinoflagellate species (Jeong et al. 2010, Lee et al. 2014). Therefore, to understand the roles of a dinoflagellate species in marine ecosystems, the species should be exactly identified.
The genus Symbiodinium, commonly known as zoo-xanthellae, comprises symbiotic dinoflagellates, most of which are symbiotic with various species such as corals, sponges, sea anemones, jellyfish, nudibranchs, clams, ciliates, and foraminifera (LaJeunesse 2002, Rodriguez-Lanetty et al. 2003, Lewis and Coffroth 2004, Fay et al. 2009, Pochon and Gates 2010, Hill et al. 2011, Pochon et al. 2012, LaJeunesse et al. in press), although some exist as free-living forms (Gou et al. 2003, Hansen and Daugbjerg 2009, Jeong et al. 2012, 2014a, 2014b). They are crucial components of coral reef ecosystems (Iglesias-Prieto et al. 1992, Stanley 2006, Stat et al. 2006); however, despite their ecological importance in marine ecosystems, not much information on their taxonomy is available. In particular, the morphological characters of Symbiodinium have been studied much less than their genetic characters (Kevin et al. 1969, Loeblich and Sherley 1979, Santos et al. 2003, Coffroth and Santos 2005, Hansen and Daugbjerg 2009, Jeong et al. 2014a) because of their difficulty in culturing and scanning electron microscopy (SEM) observations. Thus, some newly established Symbiodinium species provided only genetic information without morphological information. However, description of new species without morphological characters may cause difficulties in distinguishing species and strains in a genus. The Kofoidian plate formula has recently been used for describing a few new species from clade A including Symbiodinium natans and clade E Symbiodinium voratum (Hansen and Daugbjerg 2009, Jeong et al. 2014a).
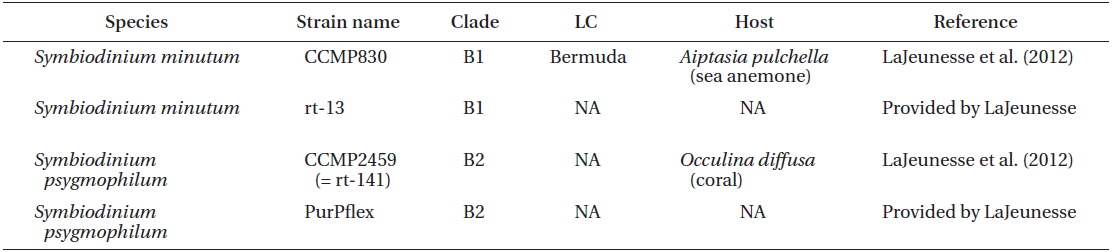
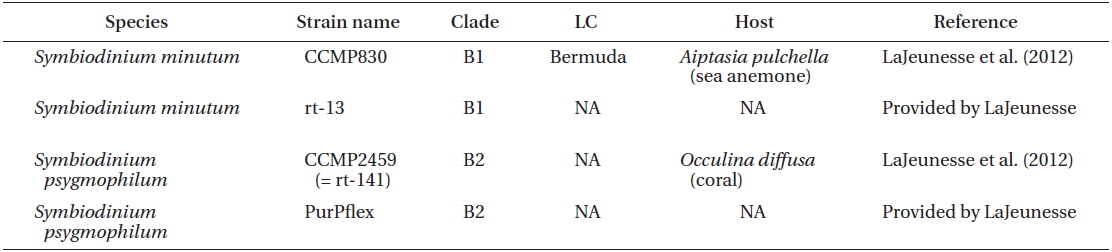
The genus Symbiodinium comprises nine clades (Rowan and Powers 1992, Pochon and Gates 2010), which are phylogenetically divergent and differ in global distributions as well as abundances (Jeong et al. 2014a). The Symbiodinium species belonging to clade B have been found inside sea anemones and corals (Rowan and Knowlton 1995, Diekmann et al. 2003, LaJeunesse et al. 2012). Until now, two species have been reported in the clade B, S. minutum and S. psygmophilum (LaJeunesse et al. 2012). S. minutum (ITS2 type B1, sensu LaJeunesse 2001) is known to form symbiotic relationships with widespread tropical anemones, including the genus Aiptasia, while S. psygmophilum (ITS2 type B2, sensu LaJeunesse 2001) is found mostly in subtropical and temperate stony corals such as Astrangia, Cladocora, and Oculina (LaJeunesse et al. 2012). However, these species were only identified by genetic markers such as nuclear ribosomal ITS1 and ITS2, single copy microsatellite flanker Sym15, mitochondrial cytochrome b (cob), and the chloroplast 23S (cp23S) rRNA gene, while their detailed morphological characterizations such as plate tabulations and related diagnoses have not been provided (LaJeunesse et al. 2012). Therefore, it is worthwhile to explore morphological characters of these two species, including plate patterns.
In this study, we analyzed the morphology of S. minutum and S. psygmophilum by SEM and transmission electron microscopy (TEM). On the basis of plate patterns, we provide complete plate formulae of these two species.
Two S. minutum strains CCMP830 from NCMA and rt-13 provided by LaJeunesse, and two S. psygmophilum strains CCMP2459 (or also called rt-141) from NCMA and PurPflex provided by LaJeunesse were used in this study (Table 1). Both species were cultivated at a temperature of 25℃ with continuous illumination of 20 μE m−2 s−1 under cool white fluorescent light and a 14 : 10 h light-dark cycle. When cultures became dense, they were transferred to new 250 mL PC bottles containing fresh L1 seawater medium approximately every 2-3 weeks. These cultures were used for genetic and morphological analyses.
The length and width of ~30 live cells from each culture in exponential growth were measured using the program ImageJ (Abramoff et al. 2004) and images obtained with a compound microscope (Ziess-Axiovert 200M; Carl Zeiss Ltd., Göttingen, Germany) at a magnification of ×1,000. Morphological analyses such as the description of the formula and shape of thecal plate of S. minutum and S. psygmophilum were performed by SEM. The cells from dense cultures of these two Symbiodinium strains were fixed for 10 min in osmium tetroxide in a final concentration of 0.3% in seawater. All other methods for SEM observation including cell collection, dehydration, drying, and observation were performed as described in Kang et al. (2010).
Intercellular observations including chromosome counting were carried out with TEM. For TEM analysis, cells of each strain with a medium were transferred to a 10 mL tube and fixed in glutaraldehyde with final concentration of 2.5%. After 1.5-2 h, tubes were centrifuged at 1,610 ×g for 10 min and the supernatants were discarded. The pellets were transferred to 1.5 mL tubes, rinsed several times with 0.2 M pH 7.4 sodium cacodylate buffer, and postfixed with 1% osmium tetroxide in deionized water for 90 min. After fixation, the pellets were embedded in agar. Dehydration was performed via a graded ethanol series (50, 60, 70, 80, 90, and 100% ethanol, followed by two changes in 100% ethanol). The agar-embedded pellet was then re-embedded in Spurr’s low-viscosity resin (Spurr 1969) and dried for 3 days at 70℃. Hardened samples were then serially sectioned (80-100 nm) using an RMC MT-XL ultramicrotome (Boeckeler Instruments Inc., Tucson, AZ, USA), and stained with 3% aqueous uranyl acetate followed by lead citrate. Finally, sectioned samples were observed with a JEOL-1010 transmission electron microscope (JEOL Ltd., Tokyo, Japan). The number of chromosomes (±standard error) in each strain was determined using these serial sections.
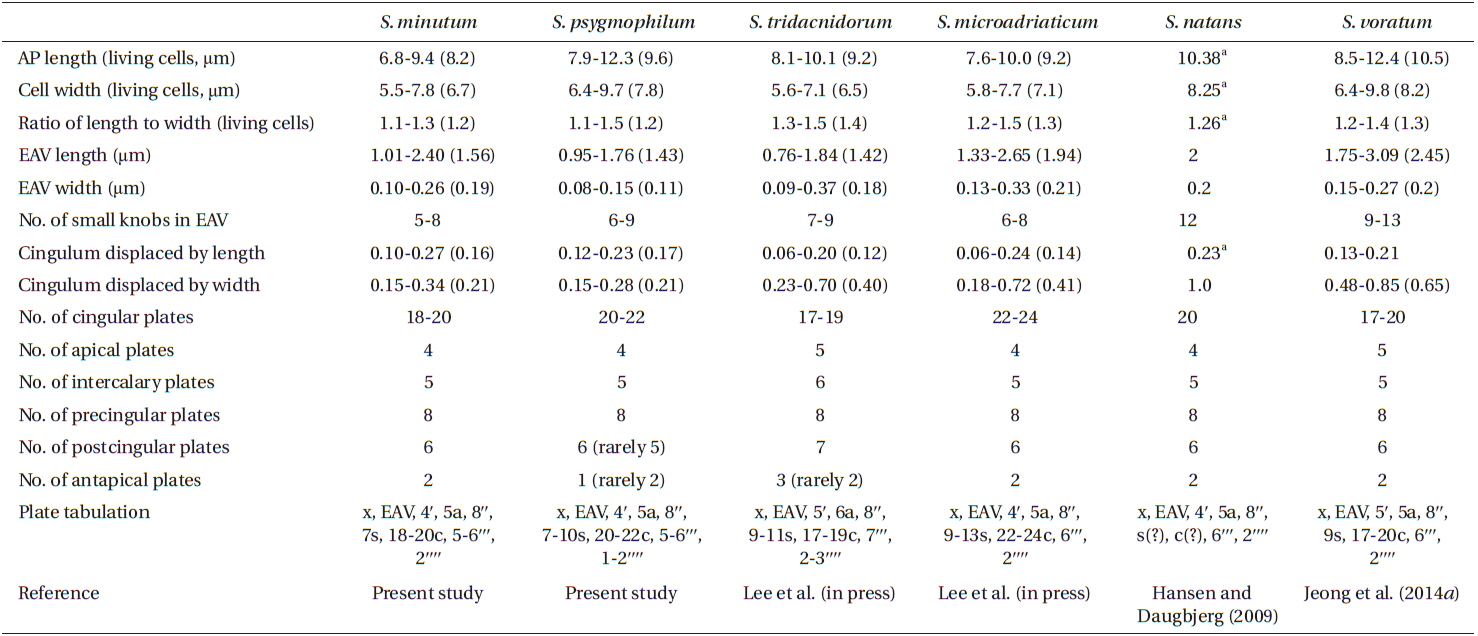
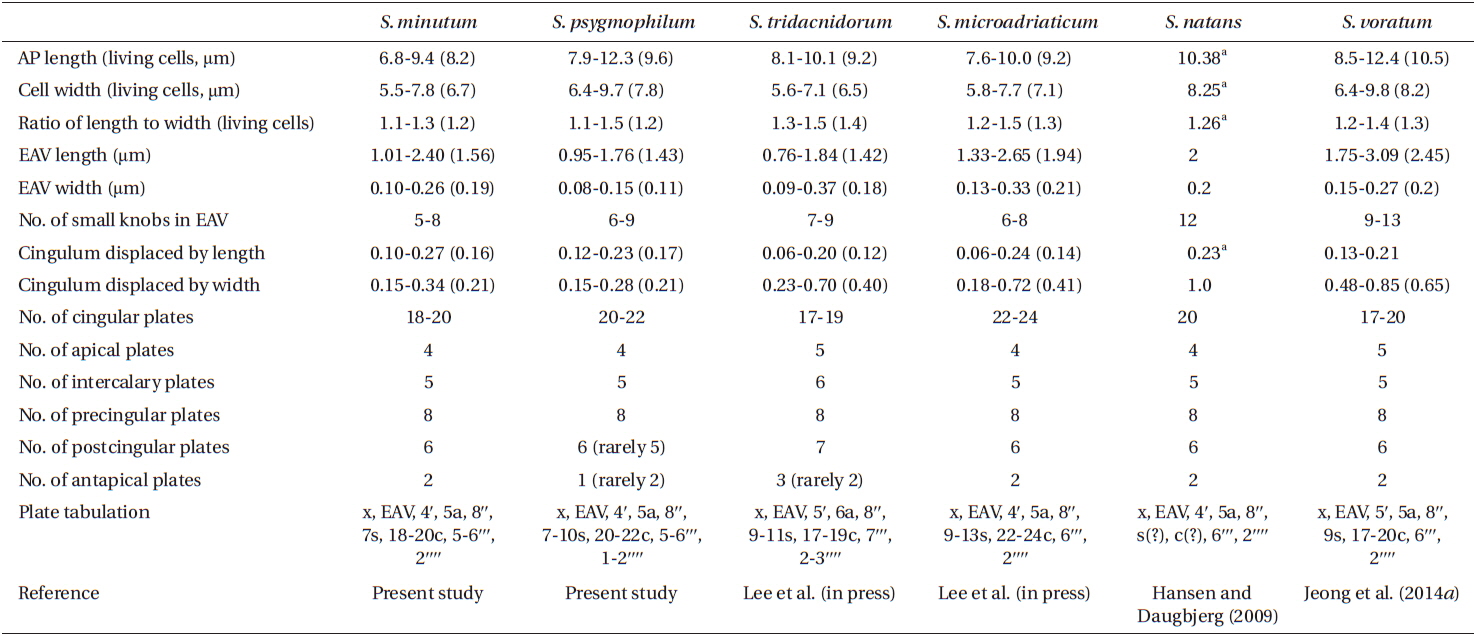
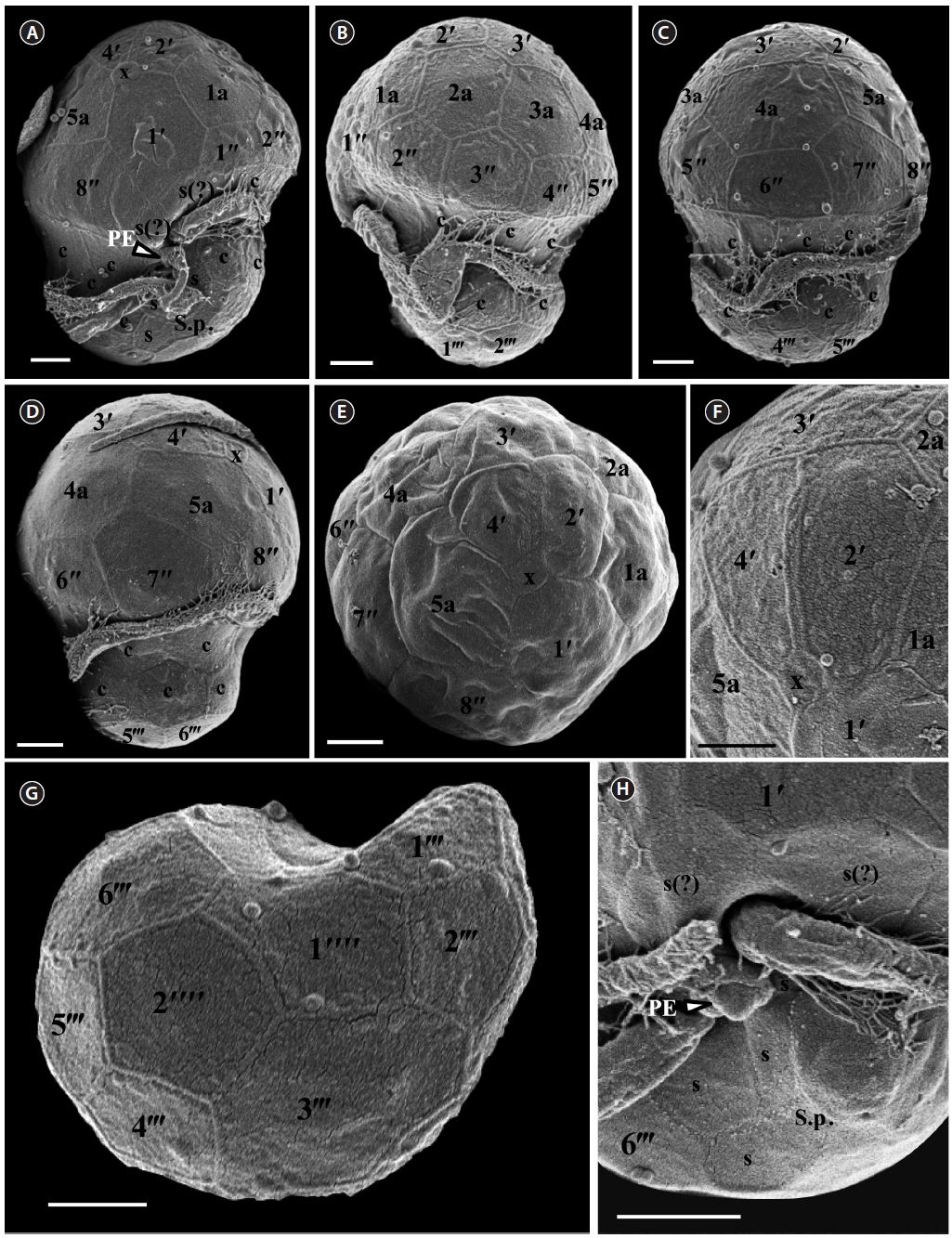
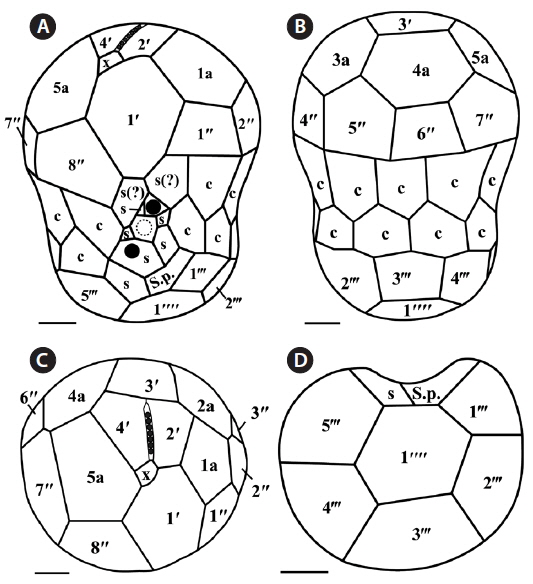
For size, approximately 30 live cells were observed and for plate tabulation analyses, approximately 150 fixed cells were observed. The size of photosynthetically grown cells observed under a compound microscope showed 6.8-9.4 μm in length and 5.5-7.8 μm in width, while the length to width ratio was 1.1-1.3 (Table 2). SEM showed that the morphologies of S. minutum CCMP830 and rt-13 were almost identical. The elongated amphiesmal vesicle (EAV) located on the apical plate was bordered by three apical plates (i.e., 2ʹ-4ʹ plates) with knobs lined up at the apex and small plate x associated in the ventral part (Figs 1E, F & 2C). The size of EAV measured under SEM showed 1.0-2.4 μm in length and 0.1-0.3 μm in width, and the number of knobs was 5-8 (Table 2, Figs 1F & 2C).
[Fig. 1.] Scanning electron micrographs of Symbiodinium minutum motile cells. (A) Ventral view showing the episome, cingulum (c), sulcus (s), peduncle (PE), and hyposome. (B) Ventral-left lateral view showing the episome, cingulum (c), and hyposome. (C) Dorsal view showing the episome, cingulum (c), and hyposome. (D) Ventral-right lateral view showing the episome, cingulum (c), and hyposome. (E) Apical view showing the episome and elongated amphiesmal vesicle (EAV). (F) Apical view showing the EAV with small knobs. (G) Antapical view showing the hyposome. (H) Antapical-ventral view showing the sulcus (s) and peduncle (PE). S.p., posterior sulcus. Scale bars represent: A-H, 1 μm.
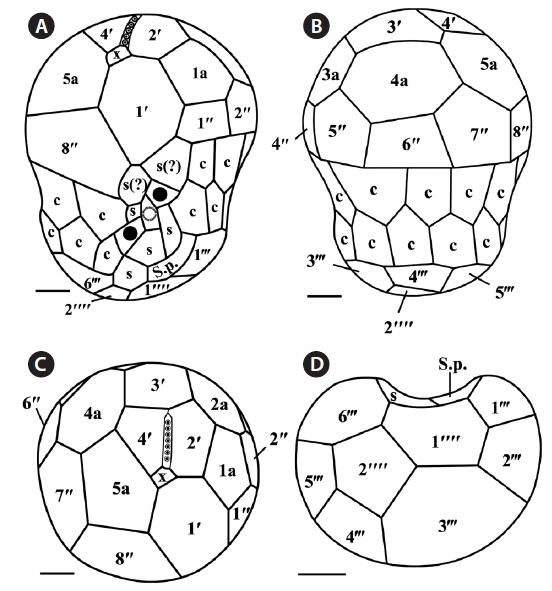
The plate formula based on Kofoidian series of S. minutum was x, EAV, 4ʹ, 5a, 8ʹʹ, 7s, two cingulum rows, 18-20c, 5-6ʹʹʹ, and 2ʹʹʹʹ (Table 2, Figs 1 & 2). The apical plates consisted of a rhomboic and relatively large plate 1ʹ and pentagonal or hexagonal 2ʹ touching plates 1ʹ, 3ʹ, 4ʹ, and 1a and 2a. The pentagonal or hexagonal plate 3ʹ touched plates 2ʹ, 4ʹ, and intercalary plates 2a-4a, whereas the pentagonal 4ʹ touched 2ʹ, 3ʹ, 4a, and 5a (Figs 1A-F & 2A-C). There were five intercalary plates: pentagonal 1a, hexagonal 2a, pentagonal or hexagonal 3a, heptagonal 4a, and pentagonal or hexagonal 5a (Figs 1B-E & 2A-C). The pentagonal plate 1a touched plates 1ʹ, 2ʹ, 2a, 1ʹʹ, and 2ʹʹ, while hexagonal 2a touched 2ʹ, 3ʹ, 1a, 3a, 2ʹʹ, and 3ʹʹ (Figs 1A, B, E & 2C). Furthermore, the hexagonal intercalary plate 3a touched plates 3ʹ, 2a, 4a, 3ʹʹ, 4ʹʹ, and 5ʹʹ (Fig. 1B). The hexagonal or heptagonal 4a always touched 3ʹ, 3a, 5a, 5ʹʹ, 6ʹʹ, and 7ʹʹ and sometimes 2ʹ, but the pentagonal 5a touched 1ʹ, 4ʹ, 4a, 7ʹʹ, and 8ʹʹ (Figs 1D, E & 2C).
The S. minutum cells had eight precingular plates that were either quadrangular (1ʹʹ, 4ʹʹ, and 6ʹʹ) or pentagonal (2ʹʹ, 3ʹʹ, 5ʹʹ, 7ʹʹ, and 8ʹʹ) in shape (Figs 1A-E & 2A-C). The cingulum was consisted of two rows of 18 pentagonal amphiesmal plates (Figs 1A-D, 2A & B). The cingulum was displaced about 0.1-0.3 times the cell length and 0.2-0.4 times the cell width (Table 2, Figs 1A & 2A).
There were six postcingular plates: 1ʹʹʹ, 2ʹʹʹ, 4ʹʹʹ, and 5ʹʹʹwere quadrangular, but 3ʹʹʹ and 6ʹʹʹ were pentagonal (Figs 1G & 2D). There were two antapical plates in hyposome: 1ʹʹʹʹ was hexagonal, while 2ʹʹʹʹ was pentagonal (Figs 1G & 2D). The 1ʹʹʹʹ contacted 1ʹʹʹ, 2ʹʹʹ, 3ʹʹʹ, 2ʹʹʹʹ, and the posterior sulcal (S.p.) plate (Figs 1G & 2D).
There were seven sulcal plates in S. minutum; four s plates, two s(?), and one S.p. (Table 2, Figs 1A, H & 2A). Furthermore, a peduncle was present in the center of the sulcal plates (Figs 1A, H & 2A).
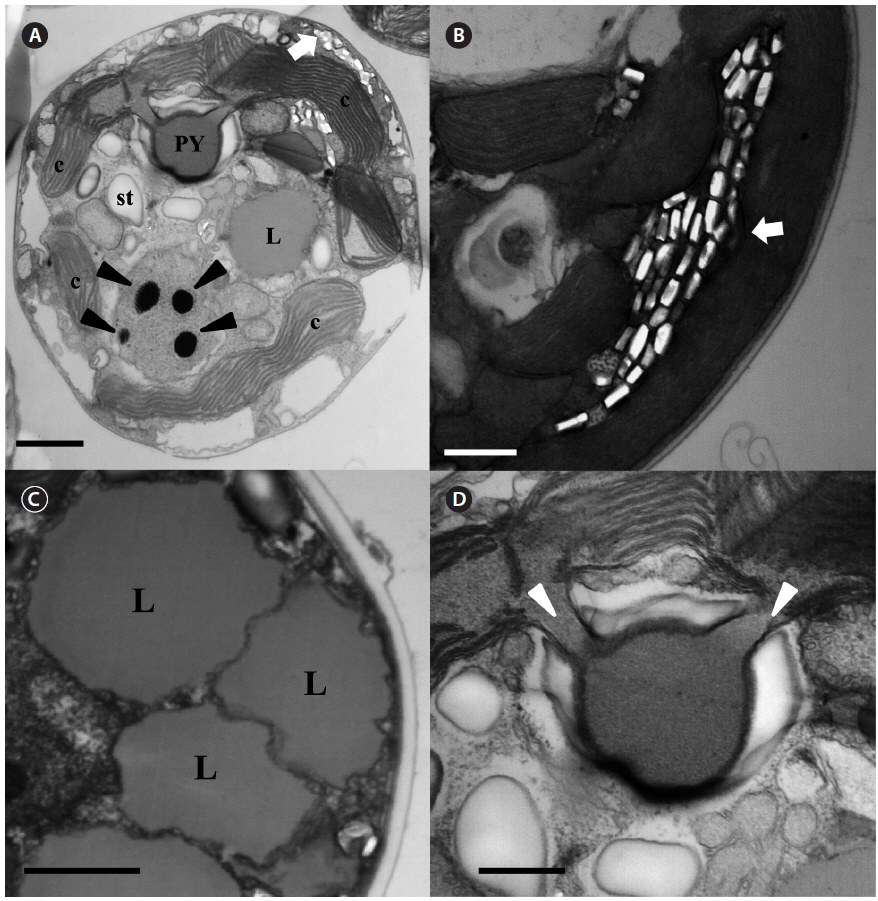
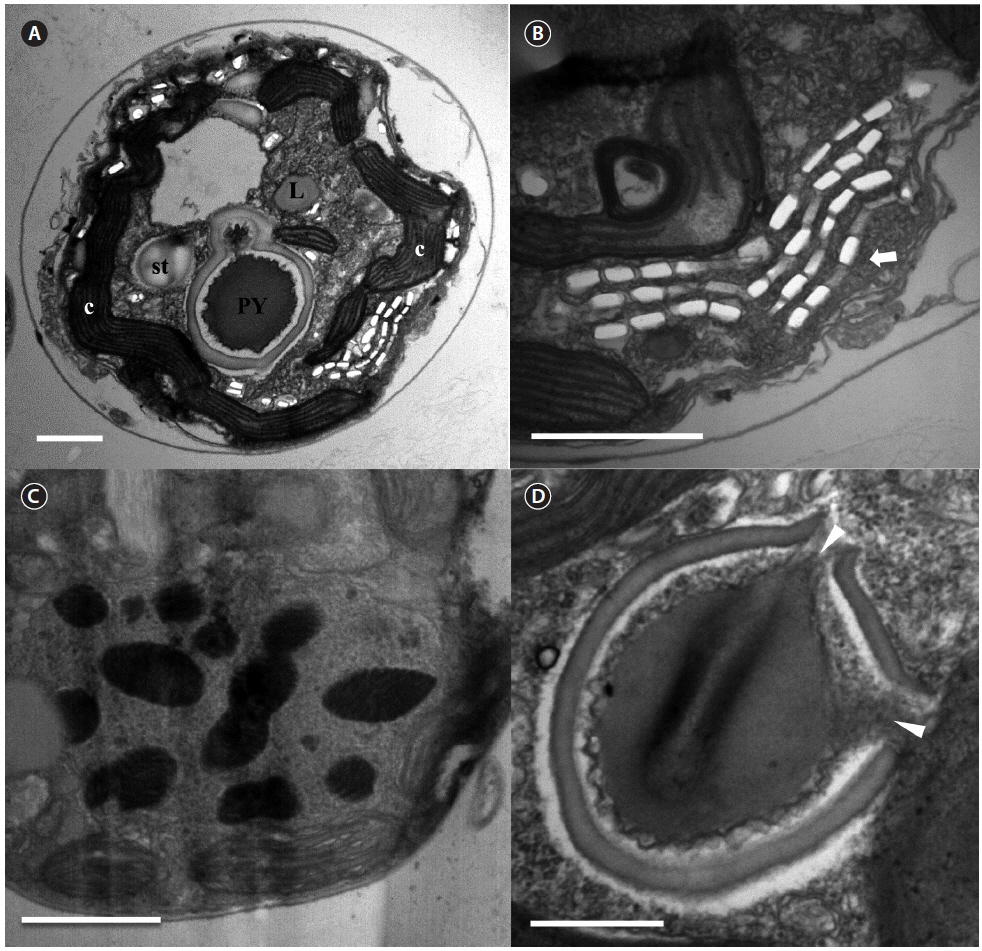
The serially sectioned TEM analysis showed that a large portion of the peripheral cytoplasm of S. minutum contained chloroplast lobes, which were connected with pyrenoid (PY) (Fig. 3A). A single PY located in the central part of each cell was connected by two stalks to the adjacent chloroplast and surrounded by a distinct polysaccharide cap (Fig. 3A & D). No chloroplast thylakoid lamellae penetrated the PY. Further, a large number of large lipid globules was present (Fig. 3C). In the sectioned cells of S. minutum, a type E eyespot consisting of multiple layers of rectangular electron-translucent vesicles or crystalline deposits was observed (Fig. 3B).
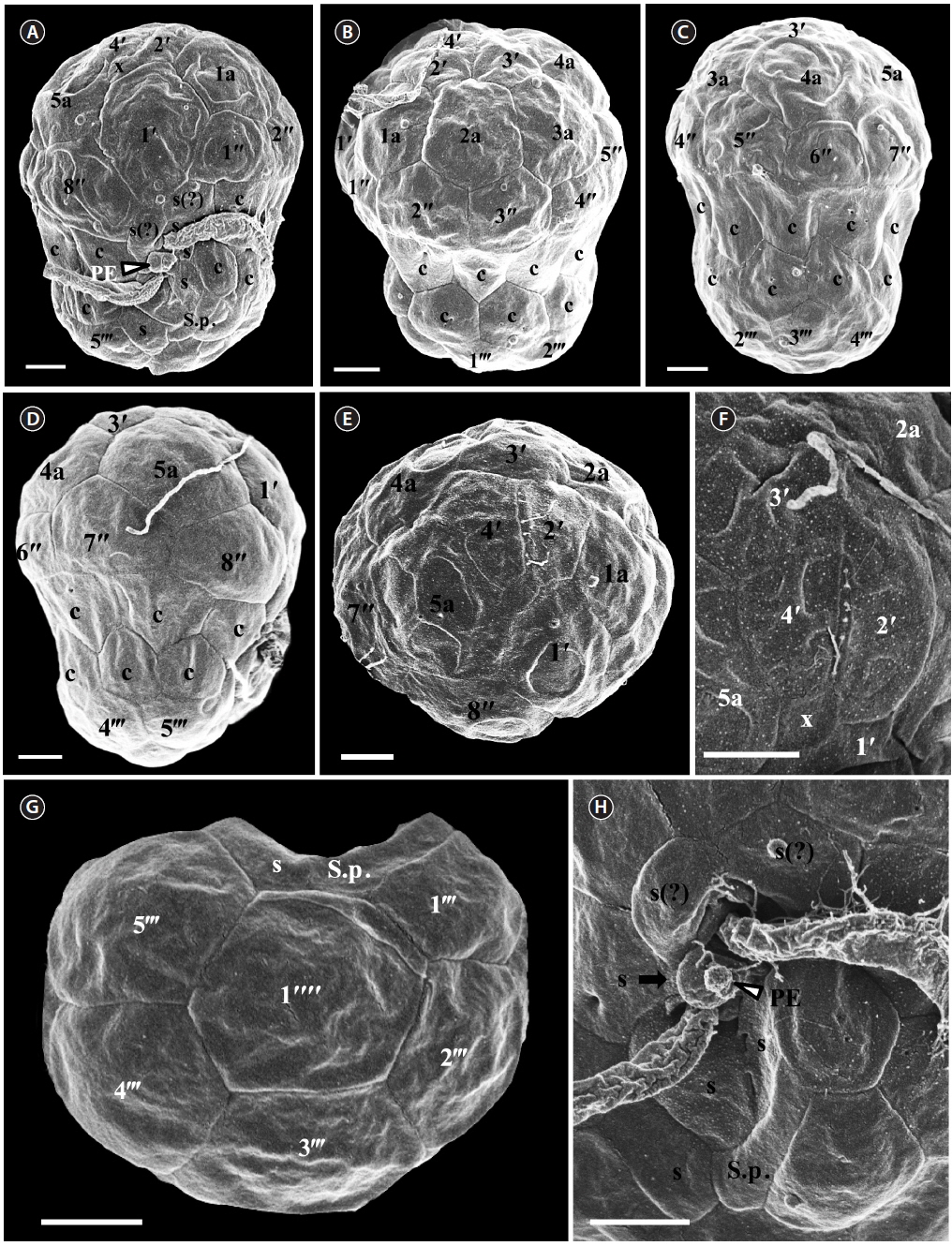
For size, approximately 30 cells were observed and for plate tabulation analyses, approximately 300 cells were observed. The size of photosynthetically grown cells observed under a compound microscope showed 7.9-12.3 μm in length and 6.4-9.7 μm in width, while the length to width ratio was 1.1-1.5 (Table 2). The SEM showed that the morphologies of S. psygmophilum CCMP2459 and PurPflex were almost identical. The EAV with knobs lined up at the apex was located on the apical plate and was bordered by three apical plates (2ʹ-4ʹ) and a small plate x associated with the ventral part (Figs 4E, F & 5C). The EAV was 1.0-1.8 μm in length and 0.1-0.2 μm in width and the number of knobs was 6-9 (Table 2, Figs 4F & 5C).
[Fig. 4.] Scanning electron micrographs of Symbiodinium psygmophilum motile cells. (A) Ventral view showing the episome, cingulum (c), sulcus (s), peduncle (PE), and hyposome. (B) Ventral-left lateral view showing the episome, cingulum (c), and hyposome. (C) Dorsal view showing the episome, cingulum (c), and hyposome. (D) Ventral-right lateral view showing the episome, cingulum (c), and hyposome. (E) Apical view showing the episome and elongated amphiesmal vesicle (EAV). (F) Apical view showing the EAV with small knobs. (G) Antapical view showing the hyposome. (H) Antapical-ventral view showing the sulcus (s) and peduncle (PE). S.p., posterior sulcus. Scale bars represent: A-H, 1 μm.
The plate formula based on Kofoidian series of S. psygmophilum was x, EAV, 4ʹ, 5a, 8ʹʹ, 7-10s, two cingulum rows, 20-22c, 5-6ʹʹʹ, and 1ʹʹʹʹ (Table 2, Figs 4 & 5). The apical plates consisted of a rhomboic and relatively large hexaor heptagonal plate 1ʹ and pentagonal plate 2ʹ touching plates 1ʹ, 3ʹ, 1a, and 2a. The pentagonal 3ʹ always touched apical plates 2ʹ, 4ʹ and intercalary plates 2a-4a and sometimes touched 5a. The quadrangular or pentagonal 4ʹ usually touched 1ʹ-3ʹ and 4a, but sometimes touched 5a (Figs 4A-F & 5A-C). There were five intercalary plates including pentagonal 1a and hexagonal 2a-5a (Figs 4A-D & 5A-C). The pentagonal 1a touched apical plates 1ʹ and 2ʹ, intercalary 2a, and precingular 1ʹʹ and 2ʹʹ (Fig. 4A, B, E, 5A & C). Furthermore, the hexagonal 3a touched 3ʹ, 2a, 4a, 3ʹʹ, 4ʹʹ, and 5ʹʹ (Figs 4B, C & 5B). The hexagonal 4a usually touched 3ʹ, 3a, 5a, 5ʹʹ, 6ʹʹ, and 7ʹʹ, while hexagonal 5a touched 1ʹ, 3ʹ, 4ʹ, 4a, 7ʹʹ, and 8ʹʹ (Figs 4C, D, 5B & C).
The precingular plates of S. psygmophilum consisted of eight plates: quadrangular 1ʹʹ and 6ʹʹ; pentagonal 2ʹʹ, 3ʹʹ, 5ʹʹ, 7ʹʹ, and 8ʹʹ; and quadrangular or pentagonal 4ʹʹ (Figs 4A-D, 5A & B). The cingulum was consisted of two rows of 22 pentagonal amphiesmal plates (Figs 4A-D, 5A & B). The cingulum was displaced about 0.1-0.2 times the cell length and 0.2-0.3 times the cell width (Table 2, Figs 4A & 5A).
There were five quadrangular postcingular plates, while there was one hexagonal antapical plate (Figs 4G & 5D). The antapical plate touched all five postcingular plates and the S.p. plate (Figs 4G & 5D).
S. psygmophilum cells possessed seven to ten sulcal plates (Table 2, Figs 4A, H & 5A): four to seven sulcal plates, two s(?), and one S.p.. A peduncle was present in the center of the sulcal plates (Figs 4A, H & 5A).
The serially sectioned TEM analysis showed that a large portion of the peripheral cytoplasm of S. psygmophilum cells contained chloroplast lobes connected to PY (Fig. 6A). A single PY located in the central part of each cell was connected by two stalks to the adjacent chloroplast and surrounded by a distinct polysaccharide cap (Fig. 6A & D). No chloroplast thylakoid lamellae penetrated the PY. A large number of lipid globules and starch were observed (Fig. 6A). Furthermore, a type E eyespot, consisting of multiple layers of rectangular electron-translucent vesicles or crystalline deposits, was observed in sectioned mastigote cells (Fig. 6B).
[Fig. 6.] Transmission electron micrographs of Symbiodinium psygmophilum cells. (A) Transverse section of a mastigote cell showing the pyrenoid (PY), nucleus, chloroplasts (c), type E eyespot (stigma), lipid globules (L), and starch (st). (B) Type E eyespot consisting of multiple layers of rectangular electron-translucent vesicles, or crystalline deposits (stigma, white arrow). (C) Cell with many chromosomes in the nucleus. (D) Single pyrenoid with two stalks (white arrowheads), located in the central part of each cell and surrounded by a distinct polysaccharide cap. Scale bars represent: A & C, 1 μm; B & D, 0.5 μm.
This study is the first report of the plate formulae and detailed morphological characters of the Symbiodinium species belonging to clade B, showing that their plate formulae are clearly different from those of the other Symbiodinium clades and also within clade B, S. minutum (type B1) and S. psygmophilum (type B2) have different plate formulae.
The plate formula of S. psygmophilum (x, EAV, 4ʹ, 5a, 8ʹʹ, 7-10s, two cingulum rows, 5-6ʹʹʹ, 1ʹʹʹʹ) is clearly different from S. natans (x, EAV, 4ʹ, 5a, 8ʹʹ, 6s, two cingulum rows, 6ʹʹʹ, 2ʹʹʹʹ) in clade A or S. voratum (x, EAV, 5ʹ, 5a, 8ʹʹ, 9s, two cingulum rows, 6-7ʹʹʹ, 2ʹʹʹʹ) in clade E, the only two Symbiodinium species with known complete plate formula (Hansen and Daugbjerg 2009, Jeong et al. 2014a). However, S. minutum (x, EAV, 4ʹ, 5a, 8ʹʹ, 7s, two cingulum rows, 5-6ʹʹʹ, 2ʹʹʹʹ) has a plate formula similar to S. natans (Table 2). Thus, this study suggests that there may be similarities in the morphologies of Symbiodinium species despite their molecular differences such as clades, or ecological differences such as hosts or locations.
Contrary to the similarity in plate formulae, S. minutum has plate shapes different from S. natans; 1a and 2a intercalary plates of S. minutum are pentagonal and hexagonal, respectively, whereas those of S. natans are hexagonal and pentagonal, respectively; the 2ʹʹʹʹ antapical plate of S. minutum is pentagonal, while that of S. natans is hexagonal. Based on the plate formulae and plate shapes, this study confirms that S. minutum and S. psygmophilum are morphologically distinct from other known species.
The postcingular 3ʹʹʹ of S. psygmophilum is rectangular in the antapical view, while S. minutum and S. voratum showed pentagonal. S. psygmophilum had a quadrangular 4ʹ apical plate, and hexagonal 4a and 5a intercalary plates, while S. minutum had a pentagonal 4ʹ apical plate and hexa- or heptagonal 4a and pentagonal 5a intercalary plates. Thus, in addition to the differences in plate formula, S. psygmophilum has plates whose shapes are different from S. minutum. Hence, the species in the same clade may have somewhat different plate shapes. Therefore, both molecular and morphological characterizations should be combined to establish a new species in Symbiodinium.
In the analysis of the ultrastructure, both S. minutum and S. psygmophilum have type E eyespot and a two-stalk PY like the other Symbiodinium species (e.g., Jeong et al. 2014a). However, S. minutum has many variously sized lipid globules, unlike the other Symbiodinium species which have only a few lipid globules (Hansen and Daugbjerg 2009, Jeong et al. 2014a). Therefore, further studies of other strains are needed to test whether this feature can be used for differentiating S. minutum from other Symbiodinium species.
To conclude, this study suggests that not all the species in a clade may have the same plate formula. Even different types within a clade, which are categorized by genetic divergences, may differ in morphology. S. minutum was originally isolated from a sea anemone, while S. psygmophilum from a stony coral. Therefore, different hosts may be related to a different morphology and genetics of the species belonging to the same clade.