The Ceramiales is the most varied order among red algae (ca. 420 genera, 2,660 species) (Guiry and Guiry 2015), but only a few genera have been found from freshwater habitats. The genus Caloglossa (Delesseriaceae, Ceramiales) is widespread in tropical to temperate mangrove, estuarine and freshwater habitats. It is comprised of 22 currently accepted species (Guiry and Guiry 2015), and the following six species have been reported from freshwater streams.
Caloglossa beccarii (Zanardini) DeToni was described as Delesseria beccarii Zanardini that occurred with Thorea flagelliformis Zanardini in a freshwater stream near Gunung Pueh in Sarawak, Malaysia (Zanardini 1872, Beccari 1904). The species is recorded as “epilithic on stones in freshwater coastal streams and epiphytic on mangrove trunks, prop roots and pneumatophores in areas with moderate water flow and high turbidity and restricted to south-east Asia (India, Burma, Malaysia, Indonesia, Singapore), the western Pacific, and northern Australia as far south as 28° S” (King and Puttock 1994, Kamiya et al. 2003). On Guadalcanal, Solomon Islands, Womersley and Bailey (1970) recorded it growing in a swimming hole near the mouth of a freshwater stream where it was subject to seawater at high tide. The first reported Indian specimen was obtained by Biswas in 1936 from the Hooghly River at the Calcutta Botanical Garden (now Ancharya Jagadish Chandra Bose Indian Botanic Garden) (http://www.nhn.leidenuniv.nl/index.php/institute).Unfortunately no herbarium specimens are available in the National Herbarium in Leiden, The Netherlands or the National Botanical Garden in Calcutta so this record is unsubstantiated. C. beccarii also has been found in other SE Asian countries including Thailand (Sato and Akiyama 2001), Vietnam (Kamiya et al. 2003) and in freshwater rivers (24-29°C, pH 7-8, conductivity 40-50 μS cm-2) in Selangor and Negeri Sembilan of peninsular Malaysia (Johnston et al. 2014).
C. beccarii, which had been recorded from a freshwater habitat in the Pédro Miguel Locks of the Panama Canal (US Alg. Type Coll. 217746) (Lin et al. 2001), was redesignated as a new species, C. fluviatilis Krayesky, Fredericq et J. N. Norris (Krayesky et al. 2012), because, in addition to the distinct phylogenetic relationship, the rhizoidal arrangement and nodal constriction of blades are distinct from those of C. beccarii (Krayesky et al. 2012).
The closely related C. ogasawaraensis Okamura was first described from the Ogasawara Islands of southern Japan (Okamura 1897) and is now widely recorded in marine, estuarine and freshwater habitats around the world (eastern USA; King and Puttock 1994, West and Zuccarello 1995) (Central and South America; Sheath et al. 1993, Pedroche et al. 1995, Krayesky et al. 2012, Zuccarello et al. 2012) (Australia, Micronesia, southeast Asia and Madagascar; Kamiya and West 2014). Caloglossa ogasawaraensis (as C. ogassuarensis Skuja) was observed in freshwater streams of Costa Rica by Soto (1982) and was first recorded from freshwater in India by Jose and Patel (1990).
Caloglossa leprieurii (Montagne) G. Martens was colthe lected from a freshwater stream in Puerto Rico (Sheath et al. 1993). In Australia C. vieillardii (Kützing) Setchell was recorded from freshwater at the Hopkins River Waterfall, Victoria and Jacksons Creek, Organ Pipes National Park, Victoria, Australia (as C. leprieurii) (West and Kraft 1996, West et al. 2001). In China C. saigonensis (as C. leprieurii var. angusta) was found in Szechwan Province near Pehpei on the Jialing River, over 1,500 km from the Yangtze River mouth near Shanghai (Jao 1941).
We have investigated the Caloglossa populations in Kerala for several years and compared them with various species using morphological, culture and molecular analyses and think it is now important to clarify general biological and taxonomic aspects of the Kerala populations.
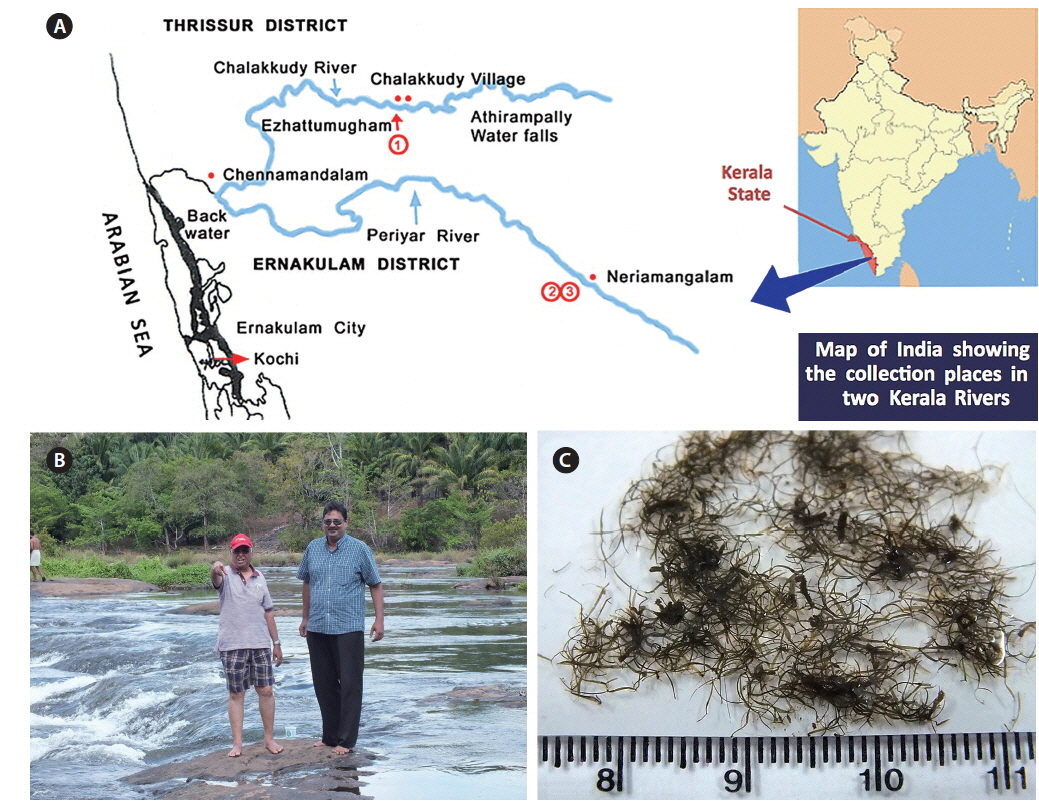
Collection details are shown in Fig. 1A map and Table 1. The Chalakkudy River general habitat and the collectors, L. Jose and E. K. Ganesan, are shown in Fig. 1B and the typical habit of a field collected plant is in Fig. 1C. Herbarium specimens of C. beccarii from Neriamangalam, Kerala are deposited in University of Michigan Herbarium (MICH 684329) and University and Jepson Herbaria, University of California, Berkeley (UC/JEPS 2050401).
Isolation and culture methods are similar to those used in earlier work (West et al. 2001, West 2005). Irradiance was 10 : 14 h diurnal light-dark, cool white LEDs or cool white fluorescent lamps (3-12 μmol photons m-2 s-1) and temperature 18-22℃ air-conditioning. Although the field specimens were collected in very low salinity (< 0.5) we cultured them in salinities of 2 or 5 with standard modified Provasoli’s medium (MPM) supplemented with peat extract to mimic tannin levels in tropical freshwater rivers. Sterile peat extract was prepared as 500-mL MilliQ water, 15 g peat moss (commercial garden package), steam sterilized for 30 min, cooled, vacuum filtered with two sheets of Whatman No. 1 (9 cm), re-sterilized and stored at 4℃. The extract fluid was clear and dark brown. One litre of culture medium was prepared as 975-mL MilliQ water, 5 mL peat extract, 10 mL seawater (salinity of 30), and 10 mL MPM nutrient enrichment. This has been useful for culturing many red algae from freshwater / low salinity habitats.
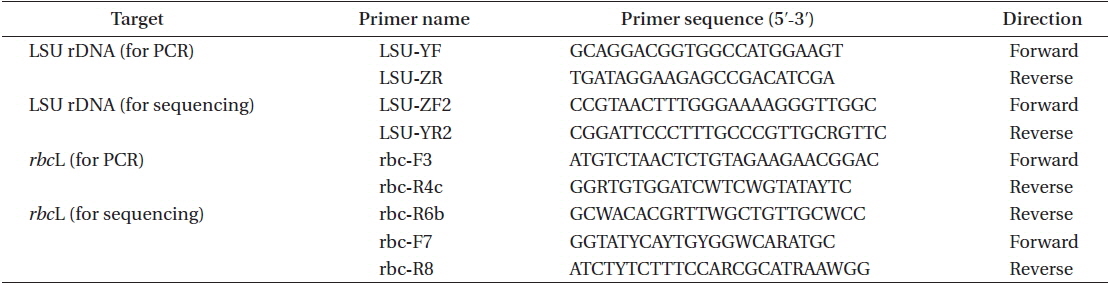
The DNA extraction method, polymerase chain reaction (PCR) conditions, and sequencing procedure followed Hayakawa et al. (2012); specifically PCR and sequencing were carried out on the large subunit of rRNA (LSU) gene and the large subunit of RuBiSCO (rbcL) gene. DNA was extracted using field-collected specimens from Chalakkudy River (India1) and from Periyar River (India2). The sequences of primers used for PCR and sequencing are presented in Table 2. The sequence data were deposited in the DNA Data Bank of Japan (http:// www.ddbj.nig.ac.jp) (LC065028-LC065031).
The methods for alignment of sequence data and construction of phylogenetic trees were the same as those described by Kamiya and West (2014). Adjustments of the resulting alignments were performed manually, resulting in 1,329 bp for LSU and 1,417 bp for rbcL. Using the program Kakusan4 (Tanabe 2011), the best-fitted substitution model was estimated for maximum likelihood (ML) analysis on the basis of the corrected Akaike information criterion (TN + G and GTR + G for LSU and rbcL, respectively) and for Bayesian inference (BI) analysis based on the Bayesian information criterion (K80 + G and GTR + G for LSU and rbcL, respectively).
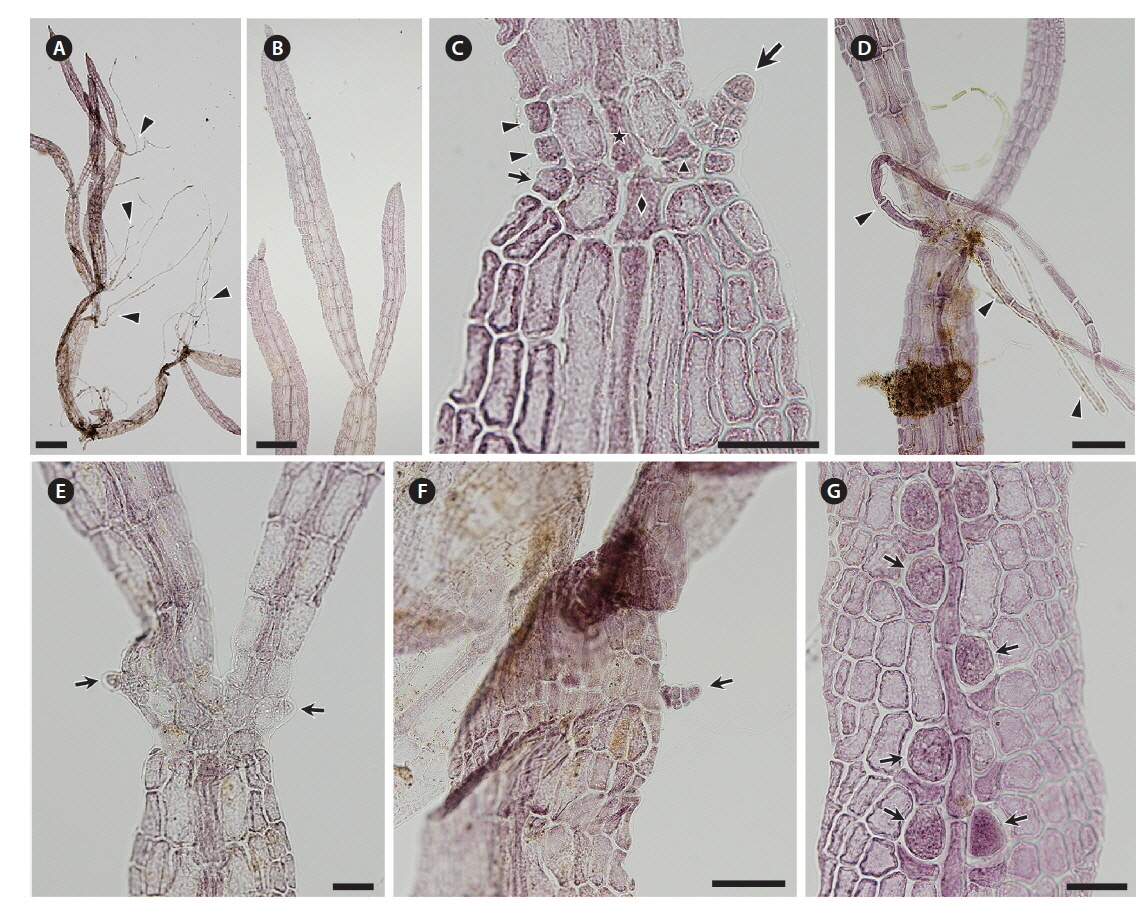
Thalli collected in the Periyar River on February 14, 2014 were growing on shallow rocks, dark red to brown, forming densely branched tufts up to 2 cm high (Figs 1C & 2A). The blades branched subapically and were linear to narrowly lanceolate, slightly constricted at the nodes, up to 4.5 mm long (Fig. 2B). Blade width was 50-160 μm at the upper part, 90-320 μm at the middle part, and 40-120 μm at the lower part. Initials of lateral axes were frequently seen not only at the first but at the second or third nodes because of their slower elongation than the main axes (Fig. 2C). The numbers of cell rows around the node, which are the important characters for identifying Caloglossa species, were as follows: one or occasionally two adaxial cell rows form from the first axial cell at the lateral axis; the nodal axial cell producing one cell row (small arrow in Fig. 2C) and the first axial cells of the main axis (FMA) forming one or two cell rows opposite the lateral blade (arrowheads in Fig. 2C). A single rhizoid filament, 20-35 μm in diameter and up to 4 mm long (Fig. 2D), was derived from a pericentral cell at the node or immediately above and below the node. No wing cells produced rhizoid filaments. Adventitious secondary blades occasionally arose from the first axial cell above the node at the same plane as that of the thallus (Fig. 2E), rarely from the marginal cells at the internodes (Fig. 2F); endogenous blades were not seen. We observed only a few plants with immature tetrasporangia (Fig. 2G). Gametophytes with sexual reproductive structures were never found in the field. All the above observations were made with dried field specimens.
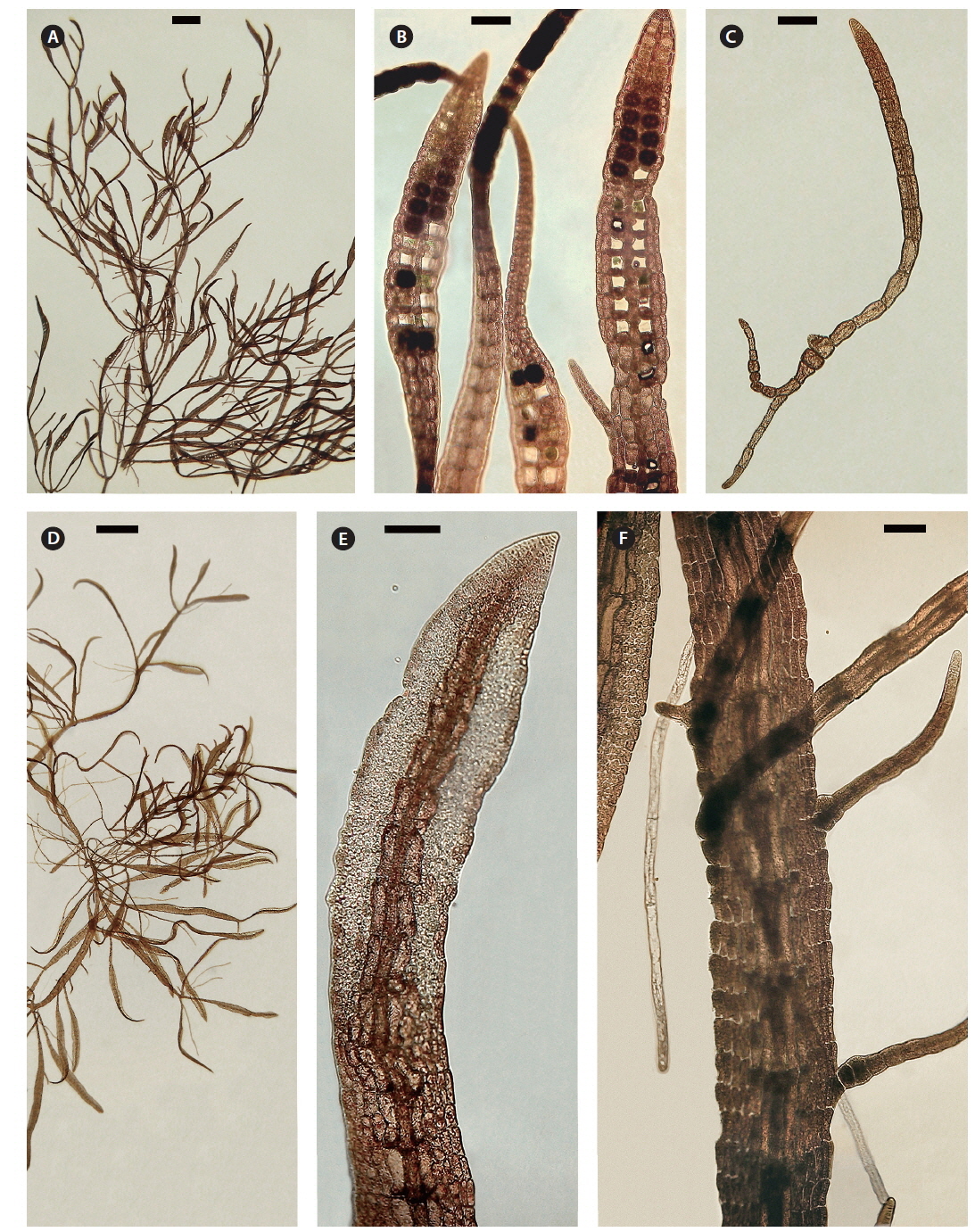
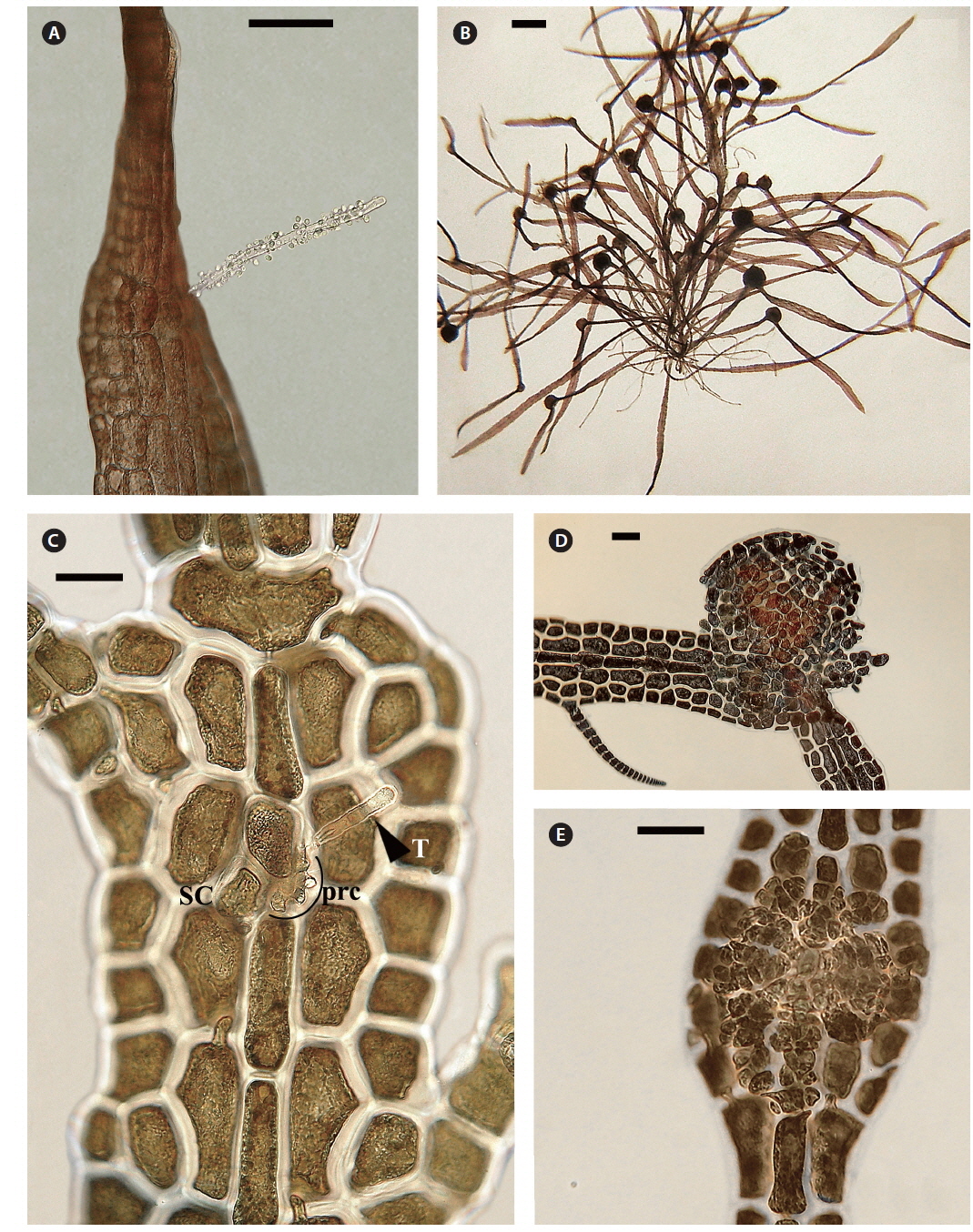
Both strains (4840 from Chalakkudy River and 4841 from Periyar River) were cultured, initially in a salinity of 5 at low photon fluence rates (3-5 μmol photons m-2 s-1) for 2-3 months and then shifted to >10 μmol photons m-2 s-1 for 4 months. Isolate 4840 grew more slowly than 4841 and did not reproduce in over one year of culture. By contrast 4841 grew robustly, plants reaching >20 mm in 3 months (Fig. 3A). After four months robust tetrasporangial sori developed (Fig. 3A & B) and many spores were discharged but germination was poor. However surviving sporelings (Fig. 3C) grew well and after 7 months male gametophytes developed spermatangial sori (Fig. 3D & E) with normal spermatia released. As in field specimens, adventitious blades of cultured blades were derived from internodal marginal wing cells of vegetative blade (Fig. 3F). Female gametophytes developed carpogonial branches along the midrib (Fig. 4A & C). Numerous carposporophytes developed in about one month (Fig. 4B & D) and discharged some carpospores from which a few sporelings developed. Also some ‘pseudocystocarps’ developed that did not form carpospores (Fig. 4E). Although at a salinity of 5 the thalli grew well and reproduced, the overall viability of spores was low. We attempted culturing field collected plants initially at a salinity of 1-2 but survival and growth were not as good as in a salinity of 5. It is puzzling that tetraspore and carpospore survival and germination were low at a salinity of 5.
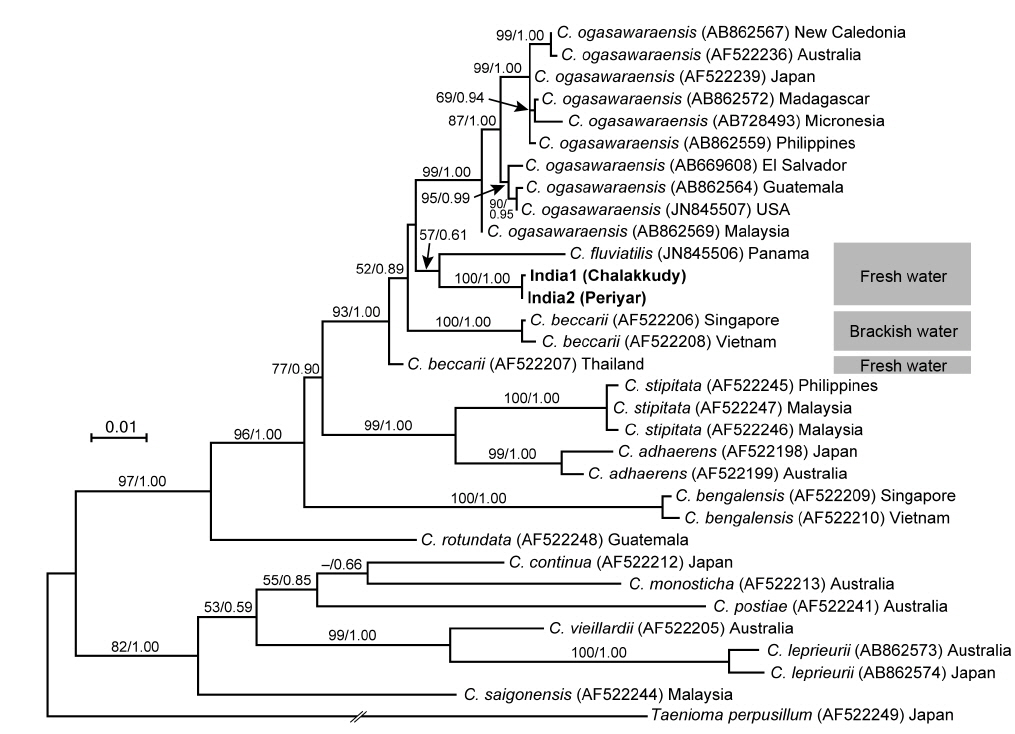
The two Indian specimens (India1 from Chalakkudy River and India2 from Periyar River) showed only one bp substitution in the LSU sequences. The LSU analyses revealed that these Indian specimens made a clade with C. fluviatilis from Panama (57% ML support and 0.61 BI support) (Fig. 5). The relationship between this clade and C. beccarii from Singapore, Vietnam and Thailand was unresolved.
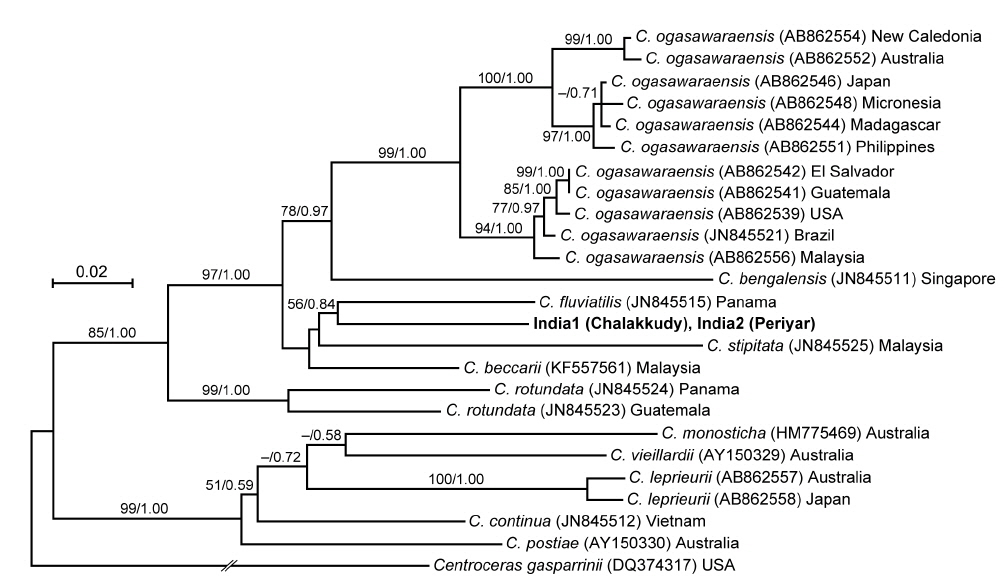
The rbcL sequences of the two Indian specimens (India1 and India2) were identical. In the rbcL phylogeny, they were also related to C. fluviatilis but it was weakly supported (56% ML support and 0.84 BI support) (Fig. 6). Their relationship with C. beccarii was unresolved.
Although the freshwater Caloglossa species are present in various temperate and tropical regions of the world neither the general biology nor molecular relationships have been investigated adequately. How did these transitions from the marine and estuarine habitats to freshwater occur? How have growth, reproduction and physiology changed in these radically different conditions? They are also subjected to a different set of cohabitants, plants, animals and pathogens. Grazing by different animals may require different defense mechanisms (Goodman and Hay 2013).
In both LSU and rbcL trees, the present alga made a clade with C. fluviatilis, which was not supported by high values of bootstrap and BI analyses. C. fluviatilis is separated from C. beccarii by forming rhizoids from nodal pericentral cells and adjacent wing cells (type F in Kamiya et al. 2003) and multiple rhizoid filaments per cell (Krayesky et al. 2012). It is occasionally difficult to delineate the arrangement pattern of rhizoids around the node, especially type D, E, and F, which are distinguished by the presence / absence of rhizoids derived form wing cells (Kamiya et al. 2003). This feature may be affected by blade width because the number of wing cells around the nodes tend to be fewer in slender thalli (Kamiya unpublished data). By contrast, the number of rhizoid derived from a cell is relatively stable and clear-cut in most Caloglossa species (Kamiya et al. 2003), and the Indian alga, which has single rhizoid filament per cell, is distinguishable from C. fluviatilis.
This alga is similar to C. ogasawaraensis based on the slender blades and the production of rhizoids only from pericentral cells around the nodes (type E in Kamiya et al. 2003). Actually Jose and Patel (1990) identified the specimen collected from Sholayar River, Kerala India as C. ogasawaraensis. However, C. ogasawaraensis produces only one cell row from FMA (Kamiya and West 2014), whereas the present alga sometimes forms two cell rows (Fig. 2C). This feature is used to distinguish C. vieillardii (mainly one cell row) from C. leprieurii (2-4 cell rows), which are phylogenetically distinct from each other (Kamiya et al. 2003). The disjunction between the Indian specimens and C. ogasawaraensis is also supported by our phylogenetic analyses.
A monophyletic relationship of C. beccarii was not supported in this study; the LSU three shows that the specimens collected from brackish water habitats in Singapore and Vietnam were distinct from that from a freshwater habitat in Thailand. Kamiya et al. (2003) carried out the detailed morphological observation of C. beccarii collected from both brackish and freshwater habitats but could not find any remarkable difference between them. Nevertheless, considering the common features, one or two cell rows numbers from FMA and single rhizoid filament per cell, the present alga should be assigned to C. beccarii. Because populations of C. beccarii examined is limited, more extensive samplings and culture experiments is necessary to delimitate this species.