This study aimed to investigate the effects of silk fibroin on bone metabolism in ovariectomized rats. A total of 30 Sprague-Dawley rats were randomized into sham-operated (SHAM), ovariectomized control (OVX), alendronate (OVX+ALEN, 10 mg/kg body weight/d), low silk fibroin (OVX+SF100, 100 mg/kg body weight/d), and high silk fibroin (OVX+SF300, 300 mg/kg body weight/d) groups. All the rats were fed by gavage for 12 wk. At the end of 12 wk, blood and urine were collected for analysis of bone turnover markers, and bone mineral density (BMD) was measured by micro-computed tomography. The results show that the OVX group (p < 0.05) displayed the highest mean body weight gain. Among the five groups, serum levels of bone alkaline phosphatase (ALP) and urine levels of deoxypyridinoline (DPD) were highest in the OVX group (p < 0.05). Bone ALP levels in the ALEN group were significantly lower than that of the silk-treated groups. On the other hand, DPD levels were not significantly different between the ALEN and silk-fibroin-treated groups (p < 0.05). The trabecular BMD was significantly higher in the ALEN and silk-treated groups compared to the OVX group (p < 0.05). In conclusion, this study showed that silk fibroin has similar effects as alendronate, which is used in osteoporosis medication. Therefore silk fibroin might be a new candidate for the prevention and treatment of osteoporosis in patients.
Bone remodeling involves sequential bone formation and bone resorption in order to maintain bone homeostasis (Kim
Rapid bone loss in menopausal women is caused mainly by estrogen deficiency, which is one of the factors responsible for regulating the production and activity of cytokines to increase bone remodeling (Recker
The long-term use of current anti-osteoporotic drugs to treat bone disease produces side effects such as nausea, headache, weight gain, breast pain, and uterine bleeding. In addition, their long-term use has contributed to increased incidence of endometrial cancer and breast cancer among patients (Andersen
Silk is a natural protein that is used commercially in textile fiber and biomedical sutures owing to its excellent mechanical strength and biological stability. Silk from the silkworm
Based on the results of previous studies, the present study administered SF to ovariectomized rats in order to closely analyze the effects of SF on menopausal bone metabolism by evaluating morphological, biochemical, and immunological markers.
Raw silk (
The Animal Care and Use Review Committee (IACUC) of Kyung Hee University approved the protocol of our experiments. A total of 30 five-wk-old female
>
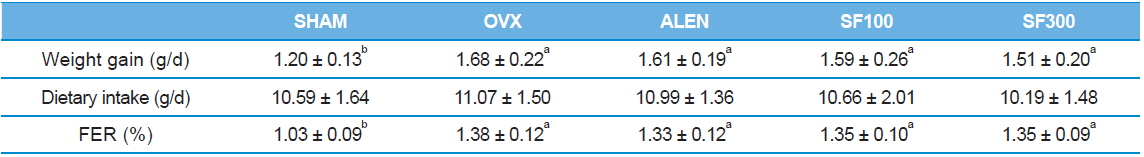
Body weight and food consumption
The body weights of the animals were measured twice per wk, and food consumption was measured daily. The food efficiency ratio (FER) was calculated using the following formula:
[weight gain(g)/d]/[amount of food consumed(g)/d].
At the end of the study, the animals were anesthetized with ethyl ether and their blood was collected by cardiac puncture. Serum was separated by centrifugation at 3,000 rpm for 15 min at 4oC, and stored immediately at -70oC until analyzed. Serum osteocalcin was measured in duplicate using MILLOPLEX bone hormone panel (Millipore, Billerica, MA, USA). Bone alkaline phosphatase (ALP) (IDS Ltd., Boldon, UK) was measured using commercially available ELISA (enzyme-linked immunosorbent assay) kits. Pro-inflammatory cytokines (IL-1β and IL-6) were measured using Millipore’s MILLIPLEX rat cytokine panel (Millipore). Measures of OPG (IDS Ltd.) and RANKL (IDS Ltd.) were obtained with Millipore’s MILLIPLEX rat bone panel 2 (Millipore).
Each animal was housed individually in metabolic cage. At 12 wk, urine was collected for 24 h from each cage. During the urine collection period, the rats were restricted from their diet to avoid urine contamination. The rats were allowed free access to water. The instruments used for urine collection were washed with 0.1 N HCl. Urine samples were centrifuged at 2,000 rpm for 15 min at room temperature, and the top layer was collected and stored at -70oC until analysis. Urinary deoxypyridinoline (DPD), which is a useful marker of bone resorption, was measured by an ELISA kit using collagen cross-linksTM (Metra Biosystems Inc., Mountain view, CA, USA).
>
Morphological analysis by μ-CT
μ-CT was performed on the left tibiae of the animals using the SkyScan 1076 (SkyScan, Kontich, Belgium) system at the beginning and the end of the experimental period. In brief, the trabecular microarchitecture together with three-dimensional (3D) images was scanned at 50 kV and 200 μA at a rotation step of 0.4o. Analyses of the reconstructed scans were also performed using NRecon cone-beam algorithm software (SkyScan), and the files were imported into CTan software (SkyScan) for 3D analysis and image generation. On the original 3D images, morphometric indices, including bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), trabecular pattern factor (Tb.Pf), structure model index (SMI), and BMD were measured.
Statistical analysis was performed using SPSS software (Version 20.0, SPSS Inc., Chicago, USA). The results were expressed as the means ± standard deviation (S.D.). One-way ANOVA was performed to determine the individual group differences. Significant differences (
>
Body weights and organ weights
The weight gain of the ovariectomized rats was significantly greater than the weight gain of the SHAM group (
[Table 1.] Weight gain, dietary intakes, and food efficiency ratio (FER)
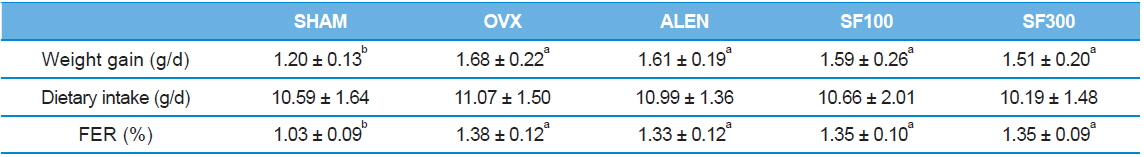
Weight gain, dietary intakes, and food efficiency ratio (FER)
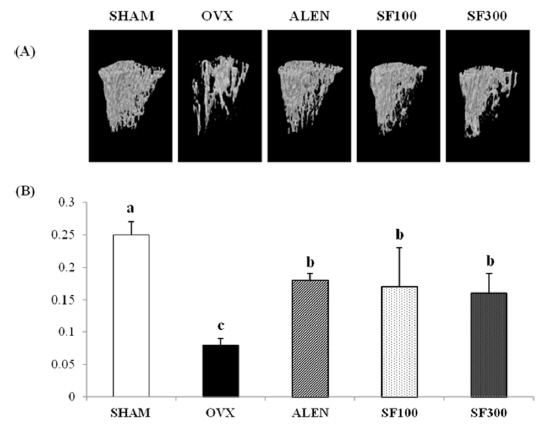
The μ-CT images showed that the microarchitecture of the animals’ trabecular bones markedly improved after the administration of SF and alendronate after ovariectomy. The BMD levels in the trabecular bones of the OVX group were significantly lower than those of the ALEN and SF (i.e., both SF100 and SF300) groups (
>
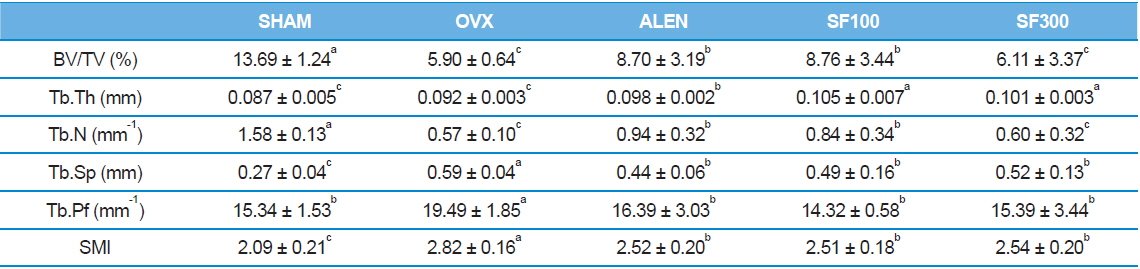
Trabecular bone histomorphometric parameters
Both the BV/TV and Tb.N of the animal trabecular bones in the SHAM group were the highest among the five groups (
[Table 2.] Trabecular bone histomorphometric parameters
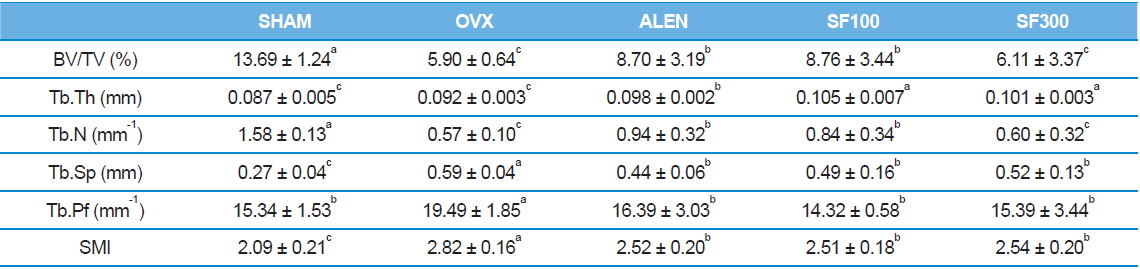
Trabecular bone histomorphometric parameters
>
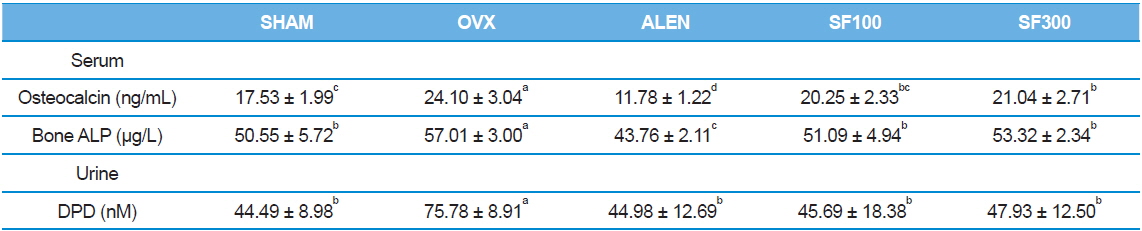
Effects of SF on biochemical parameters
Serum levels of osteocalcin and bone ALP in the ALEN group were the lowest among the five groups (
[Table 3.] Effects of silk fibroin on biochemical parameters
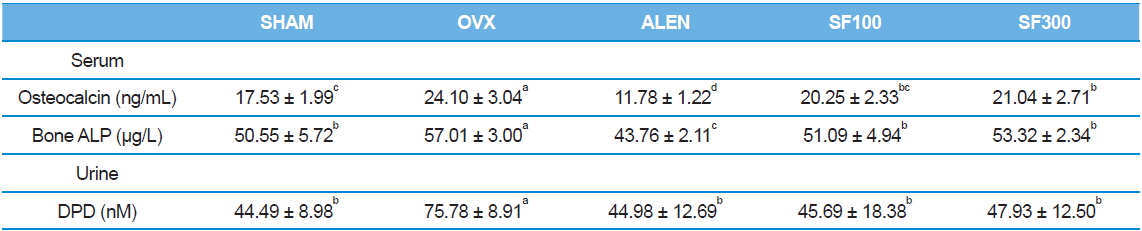
Effects of silk fibroin on biochemical parameters
>
Effects of SF on immunological parameters
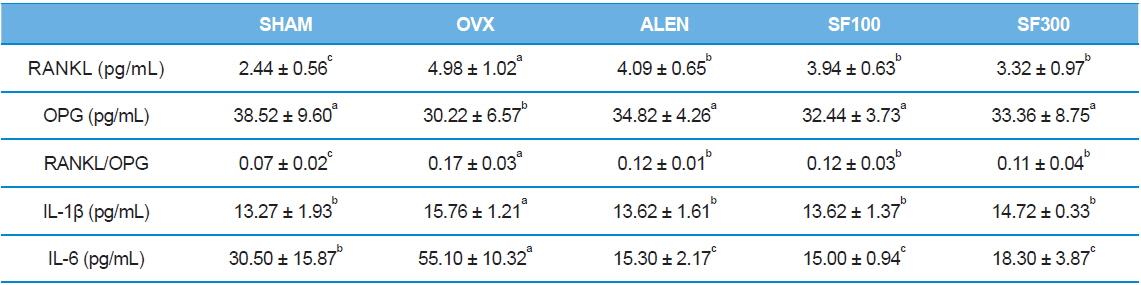
Serum levels of RANKL were significantly higher, whereas serum levels of OPG were lower, in the OVX group than the levels in the SHAM, ALEN, and SF (SF100 and SHF) groups (
[Table 4.] Effects of silk fibroin on immunological parameters
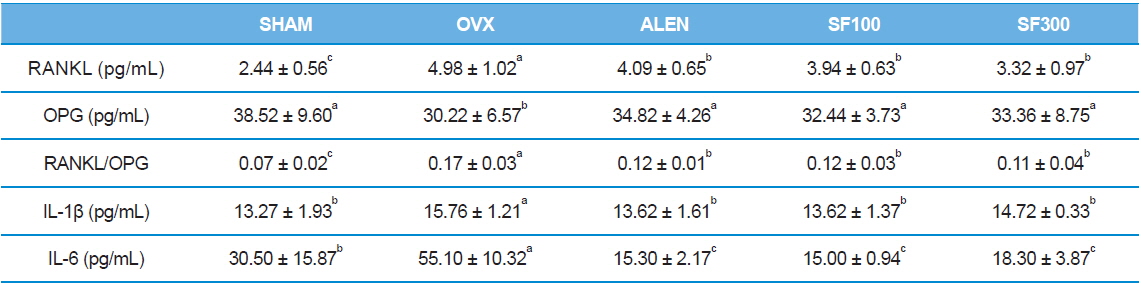
Effects of silk fibroin on immunological parameters
Menopausal osteoporosis is a bone metabolic disease characterized by low bone mass and the structural deterioration of bone tissue, which is associated with estrogen deficiency (National Osteoporosis Foundation, 2010). Because medications currently used for its treatment have various side effects, natural compounds with beneficial effects on bone have been proposed as an alternative strategy for the prevention and treatment of osteoporosis (Banu
Morphological markers of trabecular bone are helpful tools to confirm bone metabolism. Trabecular bone, one of two types of osseous bone tissue, is typically found at the end ofs of long bones, proximal to joints and within the interior of vertebrae. In many previous studies in which BMD levels were measured after ovariectomies in subjects, significant decreases were found in trabecular bone volume, whereas no significant differences were observed in cortical bone volume (Cao
Exacerbation of osteoporosis decreases BV/TV, Tb.Th, and Tb.N parameter that are directly proportional to bone mass. On the other hand, values those are inversely proportional to bone mass, including Tb.Sp, Tb.pf, and SMI increase. Thus research using μ -CT bone structure is actively underway (Lee
The above results may be attributed to changes in immunological and biochemical markers. Estrogen deficiency promotes the synthesis of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. These cytokines impact osteoclast cell differentiation and activity. In particular, IL-1 and IL-6 play important roles in mediating bone loss caused by estrogen deficiency (Canalis
Recent work has shown that RANKL, its receptor (RANK), and OPG are central players in both osteoclast development and activity. RANKL is the key regulatory factor for the differentiation and activation of osteoclasts through RANK. The biological activities of RANKL are regulated by OPG, a soluble decoy receptor for RANKL. On binding to RANKL, OPG blocks the RANKL/RANK interaction, inhibits the maturation of the osteoclasts, and consequently, interferes with bone resorption. In many situations, bone resorption is stimulated by both increased RANKL and decreased OPG, which can amplify pro-resorptive signals. Thus, it has been suggested that the RANKL/OPG ratio is the ultimate determinant of bone resorption (Liang
Serum levels of osteocalcin and bone-specific ALP are two major bone formation markers. Urinary DPD is a major bone resorption marker. Osteocalcin, an important non-collagen calcium-binding protein of the bone matrix, and ALP are synthesized by osteoblasts, bind to the extracellular matrix, and are released into the blood stream (Johnell
In conclusion, ovariectomy induces a significant increase in bone turnover, which results in dramatic trabecular bone loss. Treatment with SF, however, prevents trabecular bone loss at the tibia. Taking all the results into account, the effects of SF (not necessarily dose-dependent) were found to be similar to the effects of alendronate. That is, SF has a positive effect on menopausal bone metabolism, as shown in this study by the analysis of morphological, immunological, and biochemical markers in ovariectomized rats. Accordingly, our results strongly suggest that SF is a promising alternative for the management of postmenopausal osteoporosis in patients.