Silkworm (Bombyx mori ) feces have long been used in the pharmaceutical and food industries as a natural colorant. However, there is limited data on the bioactive compounds that constitute silkworm feces. This research emphasizes the antioxidant activity of different solvent and flavonoid extracts of silkworm feces. The solvents were ethanol, butanol, and water, while the methods utilized included ultrasonification, stirrer, reflux, and reflux after ultrasonification extraction. Results showed that butanol ultrasonification extraction (BUE) yield the lowest extraction (1.75%), while the other methods yielded 7 to 14%. The total polyphenol content utilizing BUE was 3.3 mg TAE/g, while water ultrasonification extraction (WUE) yielded the highest extraction rate with 51.6 mg TAE/g. The total flavonoid content was significantly higher using ethanol reflux extraction (EUE) at 266.8 mg QRE/g BUE, which was 158.3 and 151.3 mg QRE/g. Both DPPH radical scavenging activity and SOD-like (superoxide dismutase) activity, showed significant antioxidant effects. Finally, all other extracts except for BUE had α-glucosidase inhibition at 60%. Therefore, an effective extraction method for physiologically active substances must be selected.
Silkworms (
Silkworm feces are nontoxic, and are purported to prevent paralysis of the body and limbs, and strengthen organs. They are also effective in the treatment of skin diseases and possess anti-inflammatory and analgesic properties. Studies have shown that they are effective in the treatment of diabetes and are rich in various nutritional components (Kim
The value of utilizing silkworm feces obtained from
Utilizing various solvents and extraction methods, this study evaluated the effects of physiologically active components in dried silkworm feces. A database of effective methods to extract silkworm feces was also formed. In conclusion, the use of silkworm feces could potentially increase the value of an otherwise useless by-product of the industrial sericulture industry and raise the income of local farmers.
The silkworm feces used in this study were obtained from
>
Preparation of silkworm fecal extracts
The fecal extracts were extracted in accordance with each individual extraction protocol (listed below), the extracts were then filtered and concentrated using a vacuum evaporator. The concentrate was freeze-dried and ground into a powder. In the final step, the yield of the concentrate was investigated.
(1) Ethanol ultrasonification extraction (EUE)
The precisely weighed 100 g of silkworm fece powder was mixed with 1 L of 80% ethanol in a flask. The flask was not heated, and the extract was prepared for 2 h using 40 KHz ultrasound. This step was repeated 4 times.
(2) Ethanol stirrer extraction (ESE)
The precisely weighed 25 g of silkworm feces powder was mixed with 500 ml of 80% ethanol in a flask. Then, the contents were heated to 25±2℃ for 24 h and extracted twice.
(3) Ethanol reflux extraction (ERE)
The precisely weighed 100 g of silkworm feces powder was mixed with 1 L of 80% ethanol in a flask and then attached to a cooling tube. The extract was prepared at 60°C for 3 h in a water bath; this step was repeated 4 times.
(4) Ethanol reflux extraction after ultrasonification extraction (ERUE)
The precisely weighed 100 g silkworm feces powder was mixed with 1 L of 80% ethanol in a flask and then attached to a cooling tube. The extract was prepared at 60℃ for 3 h in water bath and then the extract was prepared for 2 h using a 40 KHz ultrasound 4 times.
(5) Water ultrasonification extraction (WUE)
The precisely weighed 100 g silkworm fece powder were mixed with 1 L of water in a flask. The flask was not heated, and the extract was prepared for 2 h using a 40 KHz ultrasound. This step was repeated 4 times.
(6) Butanol ultrasonification extraction (BUE)
The precisely weighed 100 g of silkworm feces powder was mixed with 1 L of n-butanol in a flask. The flask was not heated, and the extract was prepared for 2 h with 40 KHz ultrasound. It was repeated 4 times.
>
Determination of total polyphenol content
Total polyphenol content was determined according to the Folin-denis method (Kim
>
Determination of total flavonoid content
To measure the total flavonoid content, 0.25 mL of 0.1% (w/ v) extract was mixed with 1.25 mL H2O and 5 mL of 5% NaNO2, and allowed to react for 5 min. Then, 0.15 mL of 10% AlCl3∙6H2O was mixed into the sample, and absorbance was measured at 510 nm (Hairi
>
DPPH radical scavenging activity
Different concentrations (1.25, 2.5, 5, and 10 mg/mL) of the silkworm extracts, prepared in 96 well plates, were added to 80 μL. The resulting samples were then added to 120 μL DPPH and 6 mg ethanol, and kept in for 30 min at 37℃. Ascorbic acid was used as positive control (Kim
>
Superoxide dismutase (SOD)-like activity
Superoxide dismutase activity was measured with an aliquot of silkworm feces extract using potassium phosphate buffer in the reaction mixture. The reaction mixture consisted of 50 mM sodium/potassium phosphate buffer (pH 7.5), 58 mM nitroblue tetrazolium (NBT), 9.9 mM methionine and 0.025% (v/v) Triton X-100. Finally, 2.45 mM riboflavin was added to the mixture and allowed to react for 8 min. The color intensity was then determined at 560 nm with a UV-spectrophotometer. One unit of SOD was expressed as the quantity of enzyme required to inhibit the reduction of NBT by 50% (Almansa
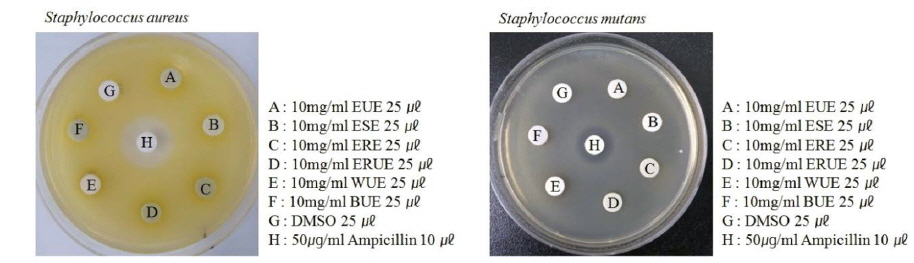
Sterilized nutrient broth was inoculated with test bacteria (
>
α-glucosidase inhibitory activity
We assayed α-glucosidase inhibitory activity using 4-nitrophenyl- α-D-glucopyranoside as a substrate. After reacting at pH 6.4 and 37℃ for 60 min, we measured the increase in absorbance at 405 nm to determine enzyme activity and to calculate inhibition.
Each experiment was carried out in triplicate, all data were the average of three independent experiments and analyzed by SPSS (version 18.0), and expressed as mean ± standard deviation (SD). Results were considered significant at
>
Total polyphenol and flavonoid contents
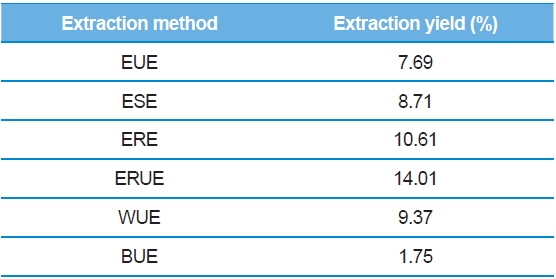
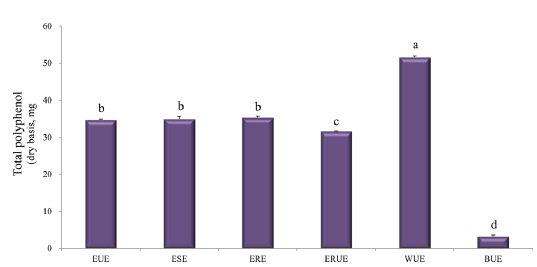
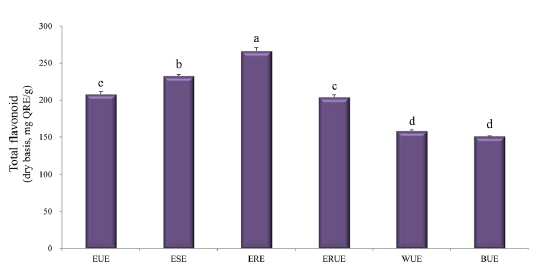
The dry matter mass was measured after freeze-drying each of extracts, and then shown as a percentage of the total material. As shown in Table 1, the yield of ethanol reflux extraction after ultrasonification extraction (ERUE) was higher than the other extraction methods. Total polyphenol contents were, analyzed by using Folin-denis method with results shown in Fig. 1. Butanol ultrasonification extraction (BUE) had the lowest results with 3.3 mg TAE/g, whereas water ultrasonification extraction (WUE) had the highest results with 51.6 mg TAE/g. The distribution of flavonoids, using different extraction methods are presented in Fig. 2. Total flavonoid contents, according WUE and BUE, were 158.3 and 151.3 mg QRE/g, respectively. On the other hand, ERE yielded a significantly high concentration (266.8 mg QRE/g). Flavonids, act as natural antioxidants and, function as anti-aging and immunity-enhancing compounds (Huang
[Table 1.] Extraction yields of silkworm feces according to extraction methods.
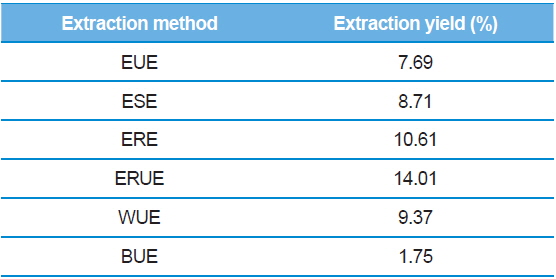
Extraction yields of silkworm feces according to extraction methods.
>
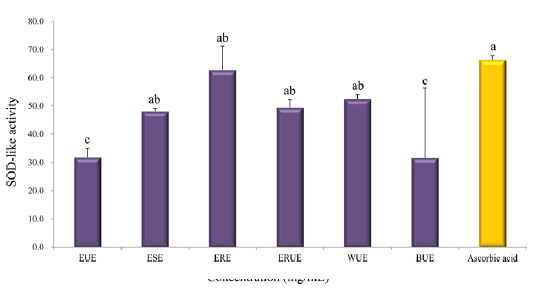
Scavenging activity on DPPH radical and SOD-like activity
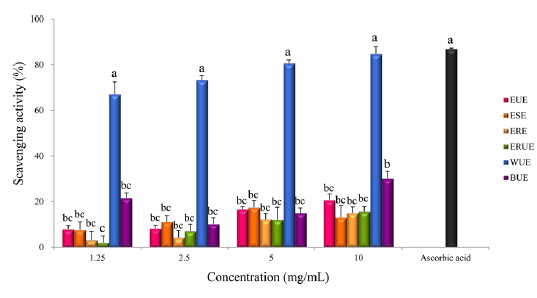
The results of DPPH radical scavenging activity showed that, there was no significant difference among the extraction methods with the exception of the WUE method (Fig. 3). Xu
This experiment compared the effects of each of the extracts on
>
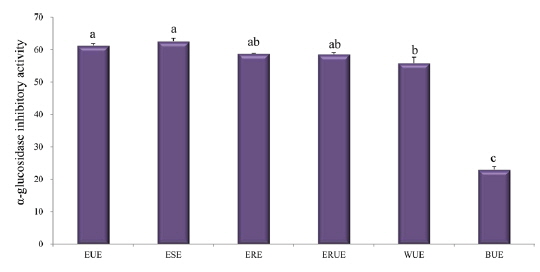
α-glucosidase inhibitory activity
For decades, researchers have shown that rat and human α-glucosidases are strongly inhibited by mulberry leaf extract (Anno