Understanding the interactions between fibroin and sericin is crucial in solving the mechanism of silk spinning. In this study, various commercially available dyes were used to monitor the interface between fibroin and sericin during the gelation of fibroin. The phase separation between fibroin and sericin could be observed by the addition of azo dyes over a certain molecular weight. Furthermore, the addition of the dyes to the sericin layer showed vivid phase separation over addition to the fibroin layer.
Silk is one of the oldest fibers known to man and has been widely used in the textile industry. Recently, a variety of advantages for silk derived products have emerged. Silk protein has excellent mechanical properties, biocompatibility and biodegradability (Kundu
The silk fiber spinning process has the advantage of using water as a solvent at room temperature and atmospheric pressure (Jin and Kaplan, 2003). Silk fiber derived from the cocoon of
A variety of dyes and dyeing processes have been developed for the use of silk in the textile industry (Tsukada
In this study, we prepared aqueous fibroin and sericin solutions laced with various dyes. We then observed the gelling behavior of fibroin that had been phase separated from sericin. Finally, an optimal dyeing system was determined for monitoring the interface of fibroin and sericin.
The silk cocoons were degummed twice with 0.2 % (w/v) sodium carbonate and 0.3 % (w/v) Marseille soap solution at 100°C for 30 min, and then rinsed with distilled water to remove any sericin. Following this, the fibroin was dried in an oven at 50°C.
>
Extraction and preparation of sericin aqueous solution
Sericin was extracted by boiling 20 g of
>
Preparation of fibroin and sericin solutions with dyes
The previously extracted fibroin was dissolved (10%, w/v) in a 9.3 M LiBr solution at 50°C for 4 h. The solution was then dialyzed in distilled water with a cellulose membrane (molecular weight cut off = 6000 – 8000, Spectrum Laboratories, Inc.) for two days. The final fibroin concentration was adjusted 5 % (w/v) with distilled water. To prepare the dyed aqueous fibroin and sericin solutions, 100 ppm of each dye was added to each solution.
>
Monitoring the gelation behavior of fibroin
The gelation experiments of fibroin were carried out as follows. First, 3 mL of aqueous fibroin was added to a 5 mL vial using a pipette. Then, 1.5 mL of aqueous sericin was added to the above fibroin solution. In order to maintain the interface between the two solutions, sericin was added carefully using a syringe pump (KD Scientific, USA). The experiments were carried out in a situation supporting a dye in either the fibroin or sericin solutions.
During the silk fiber spinning process, sericin envelopes two strands of fibroin. However, the role of sericin in covering fibroin is still unclear. Oh
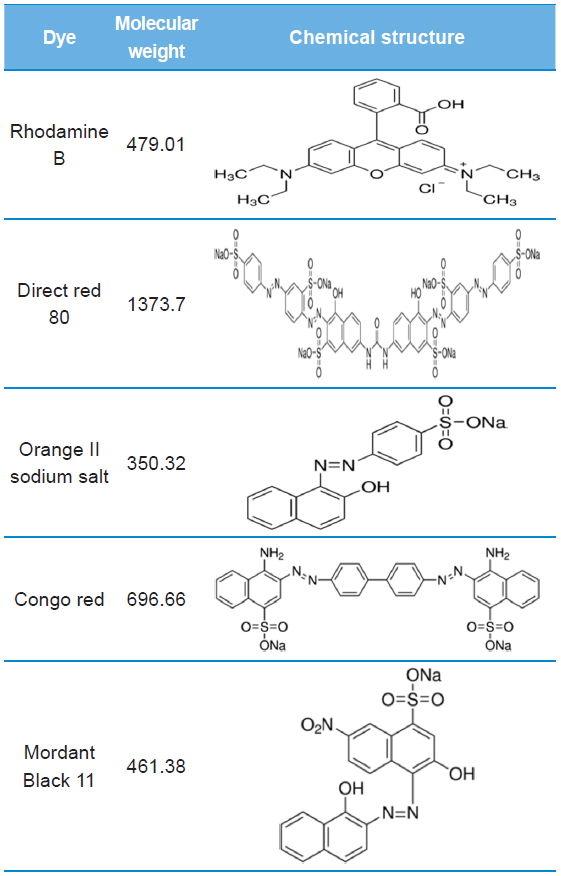
[Table 1.] Molecular weight and chemical structure of dyes using this study
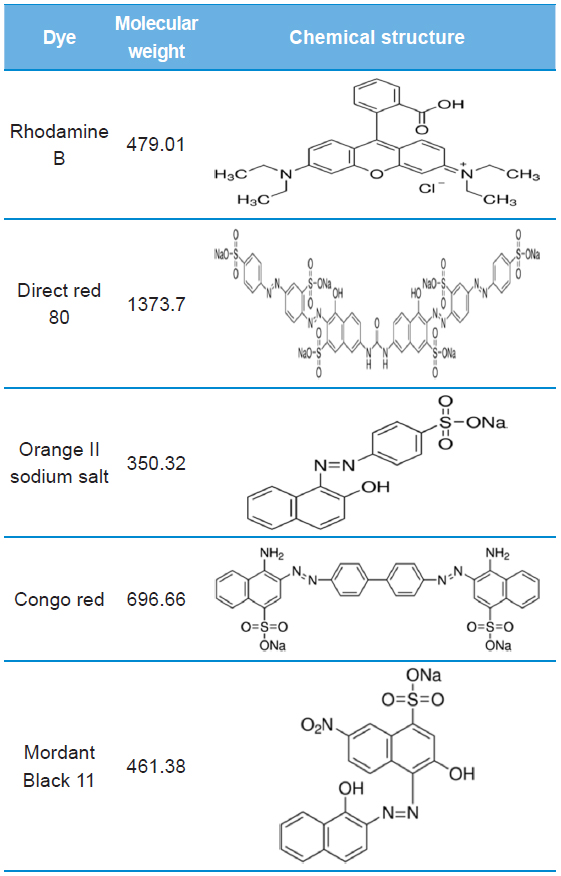
Molecular weight and chemical structure of dyes using this study
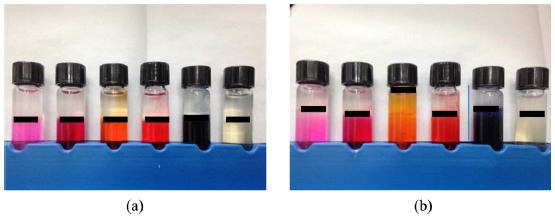
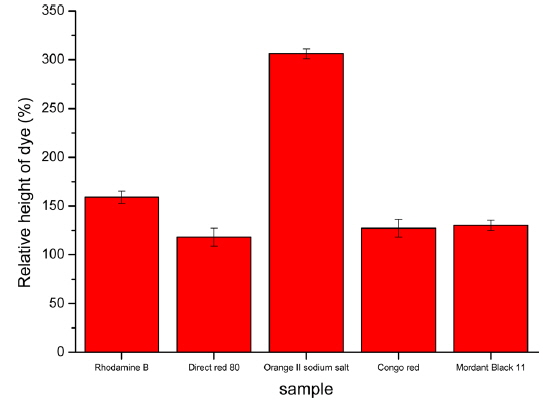
To efficiently observe the interface between fibroin and sericin, we performed similar gelation experiments using dyed sericin aqueous solutions. The phase separation between the fibroin and sericin solutions before and after gelation and the relative height of the dyed solution after gelation compared to its initial height are shown in Figs. 3 and 4. Similar to the above experiment, when using direct red 80, Congo red and mordant black 11 dye, phase separation between sericin and fibroin solutions was well maintained. In the case of rhodamine B and orange II sodium salt, diffusion of dye from the sericin to the fibroin layer was observed. This could also be explained by the difference in molecular weights of the dyes and the cationic structure of rhodamine B. The interface between the two proteins is easier to observe when the dye is added to the sericin solution. The addition of dyes to the sericin layer did not show a large difference between the original and final location of interface. When the dyes were added to fibroin, movement of the dye into the sericin layer is relatively easy since sericin does not gel at 50°C. Conversely, when dyes were added to sericin, fibroin aqueous solution generates a three-dimensional polymer network during the gelation. This gel structure is to interfere with the penetration of the dye and facilitates the observation of the interface between fibroin and sericin.
In summary, the interface between fibroin and sericin could be monitored using azo dyes with a molecular weight high enough to prevent diffusion between the protein layers. It was also confirmed that when dyed sericin solutions were used, a more vivid interface was observed. This technique for monitoring the interface between fibroin and sericin using dyes could be helpful in understanding the interactions between these silk proteins.