A new species of the genus Farranula Wilson, 1942 (Cyclopoida, Corycaeidae) is described based on both sexes collected off Chuuk Island in Micronesia (West Central Pacific). The new species F. dahlae differs from its close congener F. gibbula (
The family Corycaeidae Dana, 1852 includes two genera,
However, recent detailed morphological examination and genetic analysis of many of the alleged cosmopolitan species of small cyclopoid copepods, such as oncaeids, have proven to be species complexes, which comprise two or more closely related species (Wi et al., 2010, 2012; Böttger-Schnack and Machida, 2011). In addition, since previous studies of most species of
In this study, we confirm that the material from Chuuk Island represents a new species and provide a full description of this new species. In addition, some comparisons of morphological characteristics between the new species and
Zooplankton was taken from off Chuuk Island in Micronesia, on 12 June 2012 (Fig. 1A). A conical net (mesh size 100 μm, mouth diameter 45 cm) was towed vertically from near bottom to the surface. According to the vertical CTD profiles (Sea-bird Electronics, Inc., Bellevue, WA, USA), a warmer water mass (>28℃) was found above 50 m, and the maximum salinity layer (>35 psu), with a thickness of ca. 50 m was located at a depth of 150-200 m. After collection, samples were immediately preserved on board, in a 4% formaldehyde-seawater solution buffered with borax. Specimens of the new species were sorted out for study. Material of
Females of
Order Cyclopoida Burmeister, 1835
Family Corycaeidae Dana, 1852
Genus Farranula Wilson, 1932
Key to the females of
1. Urosome evenly tapering posteriorly, with proximal expansion both in dorsal and lateral views ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙2
Urosome with undulating lateral margins, with proximal expansion on dorsal surface in lateral view ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙3
2. Eyes adhered to each other ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙4
Eyes located at some distance ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙5
3. Second prosomal somite with dorsal hump ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙6
Second prosomal somite without dorsal hump ∙∙∙∙∙∙∙∙∙∙∙∙∙7
4. Second urosomal somite less than 3 times length of caudal rami ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
Second urosomal somite more than 4 times length of caudal rami ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
5. Anterior margin of second urosomal somite ventrally protruded; second prosomal pointed margin reaching more than 70% distance of second urosomal somite ∙∙∙∙∙
Anterior margin of second urosomal somite with ventral protrusion; second prosomal pointed margin reaching less than 40% distance of second urosomal somite ∙∙∙∙∙
6. Second urosomal somite less than twice length of caudal rami ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
Second urosomal somite more than twice length of caudal rami ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙8
7. Second urosomal somite sharply tapered from mid-region in lateral view ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
Second urosomal somite gradually tapered in lateral view ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
8. Caudal rami more than 5 times length of wide ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
Caudal rami less than 4 times length of wide ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙
Material examined. Holotype: adult female dissected and mounted on 2 glass slides, MABIKCR00218002; collection from waters adjacent to Chuuk Island, Micronesia (West Central Pacific) (16°17′054N, 134°58′200′′E) on 12 June 2012.
Paratypes: two adult females and two adult males dissected and mounted on 2 glass slides, MABIKCR00218003 and 3 glass slides, MABIKCR00218004: collection from waters off Chuuk Island, Micronesia (West Central Pacific) (16°17′054N, 134°58′200′′E).
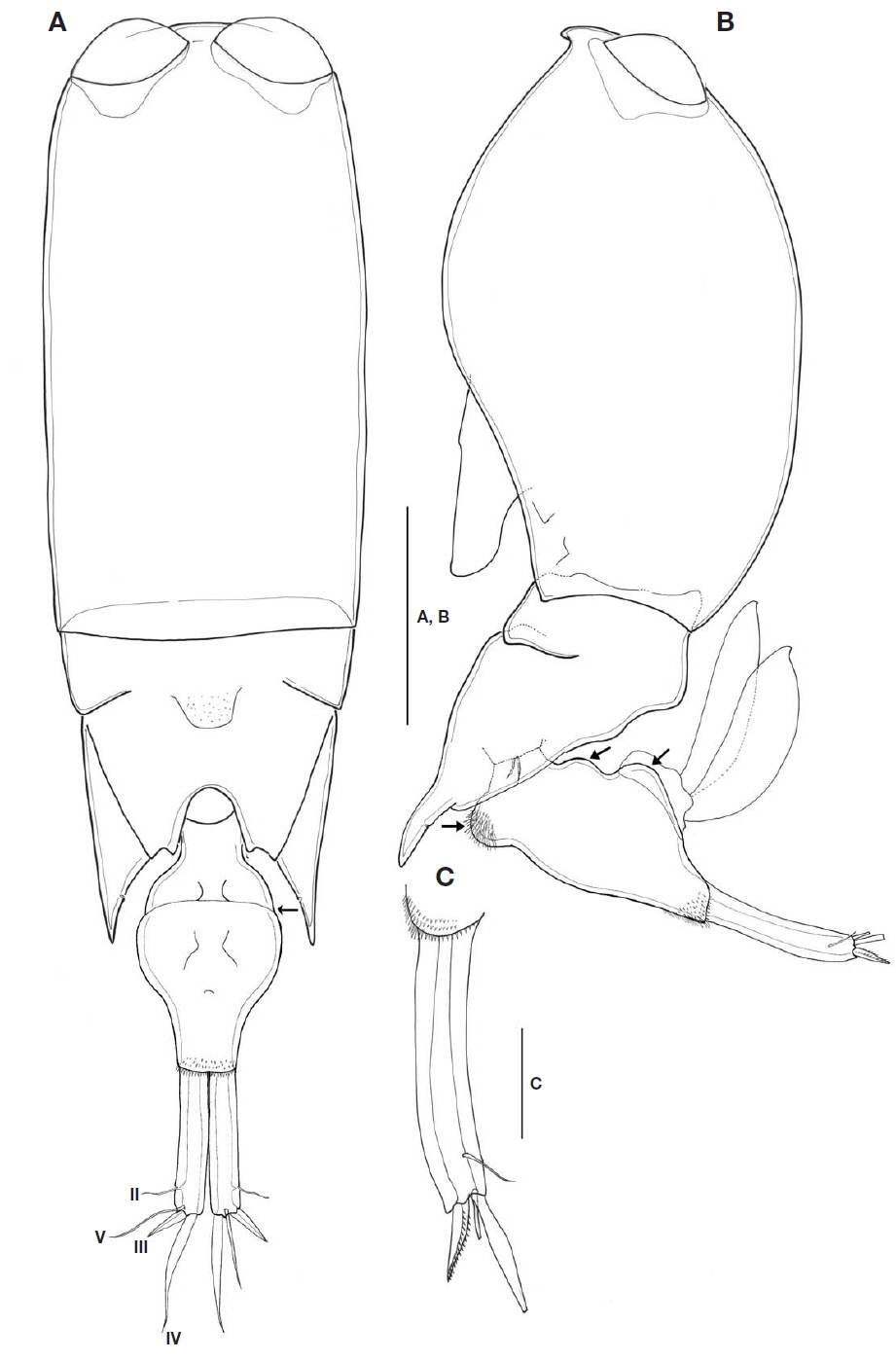
Description of female. Body cylindrical, tapering posteriorly. Total body length in lateral view 1,011-1,031 μm(n=3). Urosome distinctly narrower than prosome.
Prosome two-segmented (Fig. 2A, B), frontal part rounded, with two large separate cuticular lenses: cephalosome completely fused with first pedigerous somite, with ventral process in lateral view, 1.6 times longer than wide, second to fourth pedigerous somites forming single compound somite with suture line between second and third pedigerous somites, prosome about 2.1 times as long as urosome including CR, 3 times as long as urosome excluding CR; third pedigerous somite dorsally covering fourth pedigerous somite, forming inverted triangular shape; fourth pedigerous somite extending almost to middle of second urosomal somite, with pointed posterolateral corners, secretory pore on inner pleural area.
Urosome (Fig. 2A, B) two-segmented: first urosomal somite bearing P5 ventrolaterally; genital compound somite and anal somite combined. Proportional lengths (%) of urosomites and caudal rami 4.4 : 59.6 : 36. Second urosomal somite (Fig. 2A, B) irregular, showing folds at anterior third in dorsal view (indicated by arrow in Fig. 2A), lateral greatest width, 1.8 times longer than maximum width at mid region, protruded with rounded hump on anteroventral margin (visible in lateral view and arrowed in Fig. 2B), bearing patch of spinules, posteroventral and lateral margins fringed with minute denticles; dorsal and ventral surfaces covered with small denticles, posterodorsal surface usually with 2 attached spermatophores (Fig. 2B); genital area located dorsolaterally, paired genital apertures at mid-region of dorsal surface, hidden behind opercula.
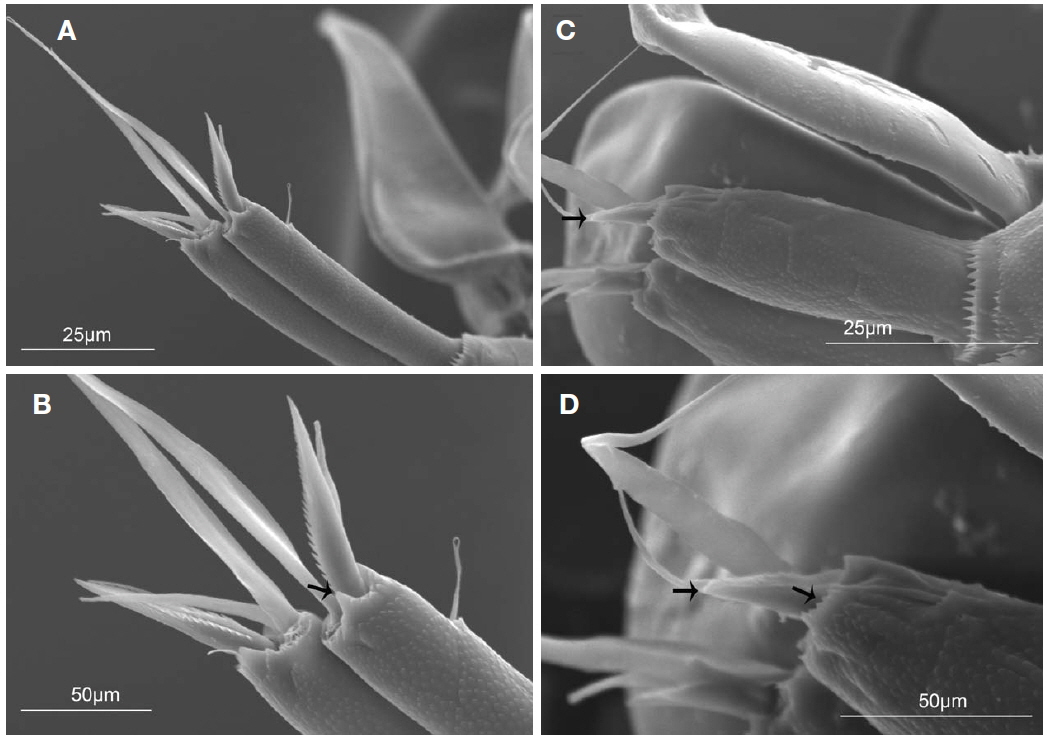
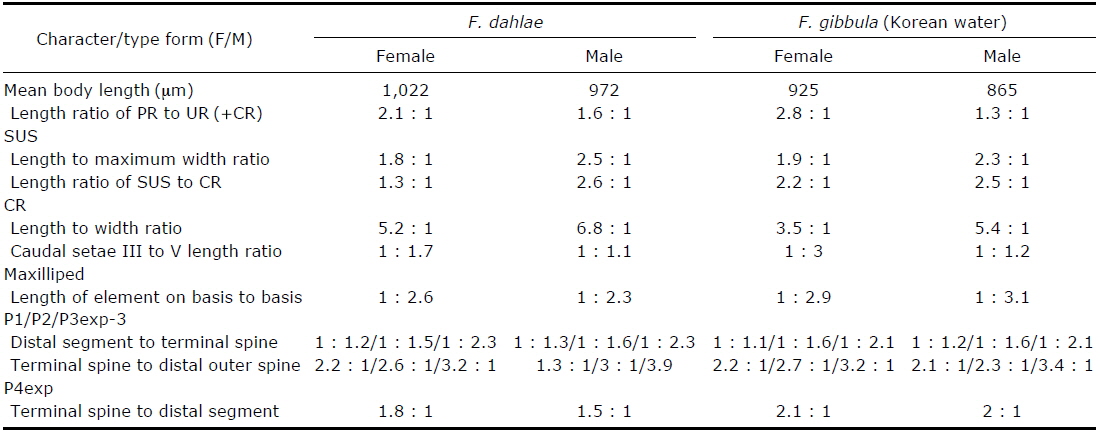
CR (Figs. 2A, C, 5A, B) cylindrical, 0.6 times as long as second urosomal somite, 5.2 times longer than width at base. Each ramus with triangular process located near insertion and armed with four setae (arrowed in Fig. 5B): slender anterolateral seta II, bipinnate posterolateral seta III short and robust, inner terminal seta IV longest, and dorsal seta V 1.7 times longer than seta III. Entire surface ornamented with small denticles (Fig. 5A).
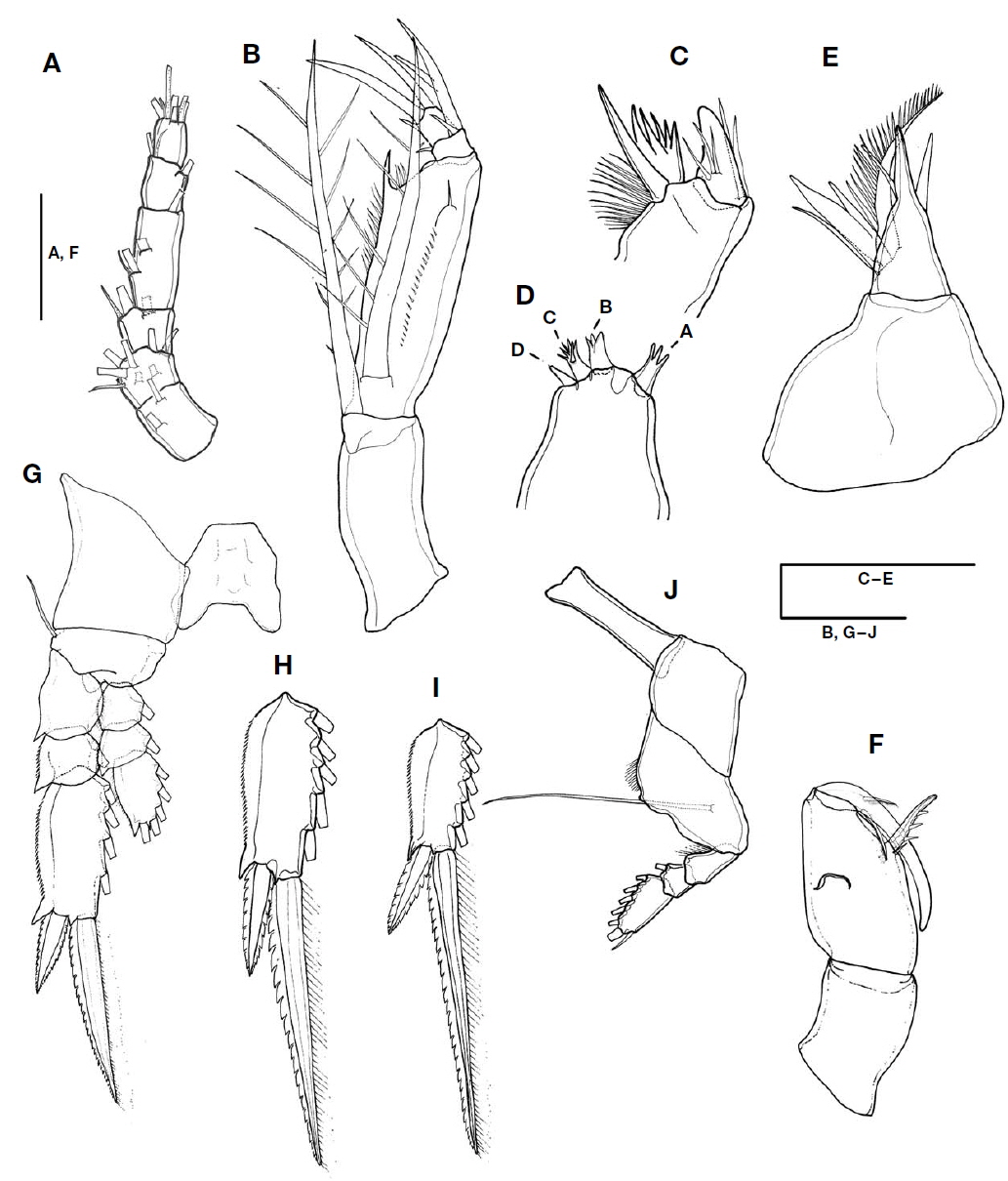
Antennule (Fig. 3A) short, 6-segmented. Armature formula 1-[2], 2-[8], 3-[2+ae], 4-[3+ae], 5-[2+ae], 6-[5+(1+ae)]. Proportional lengths (%) 20.7 : 8.8 : 13.2 : 29.4 : 14.7 : 13.2.
Antenna (Fig. 3B) 4-segmented with coxa and basis fused. Coxobasis 1.9 times longer than width at base, with strong seta at distal margin. Endopod three-segmented: first endopodal segment robust, 4.7 times longer than remainder of endopodal segments, 3.7 times longer than width at base, bearing bipinnate seta on inner proximal margin, about 0.9 times as long as coxobasal seta; inner distal margin with long spinelike process, outer lateral margin ornamented with row of denticles, naked seta on distolateral margin; second endopodal segment shortest, bearing three elements: curved stout spine arising from outer distal margin, with lateral branch; slender, pinnate spine located near base and reaching almost one-third of distal spine; short curved spine arising from inner margin; outer distal spine 2.3 times longer than branched spine; third endopodal segment cylindrical, as long as wide, and armed with three elements: one stout terminal spine, short spine arising from inner margin, strong seta on outer margin; terminal spine equal to outer spine on second endopodal segment in length. Length ratio of terminal spine to outer spine on third exopodal segment 1.4 : 1.
Mandible (Fig. 3C) with two elements on gnathobase: one spine and one blade; spine broad and robust, with three naked slender setules on medial area and two basal setules; blade forming spiniform processes, surrounded by patch of spinules around base.
Maxillule (Fig. 3D) reduced, praecoxal arthrite bearing four articulated spinous elements: innermost element (A) separated from other elements, trifurcate, slightly longer than element B; element B stout, distally trifurcate, as long as element C, distal margin of element C serrated, and element D shortest.
Maxilla (Fig. 3E) two-segmented, allobasis shorter than syncoxa: syncoxa unarmed; allobasis produced distally into strong spine, with four naked setae proximally, inner margin bearing three spines of different lengths: medial one naked, ventral one split into two branches, and dorsal one longest, unipinnate innermost spine.
Maxilliped (Fig. 3F) three-segmented: syncoxa unarmed; basis robust and expanded, 2.2 times longer than width at base, with two elements along inner margin: proximal element short, located at base of distal element; distal element with three spinules along inner margin, located at anterior two-thirds distance of inner margin, 0.38 times as long as basis; endopodal segment drawn out into long curved claw, unornamented and much shorter than basis, accessory armature consisting of long slender, unipinnate seta on inner proximal margin, and short unilaterally pectinate spine laterally on outer proximal margin.
Swimming legs 1-3 (Fig. 3G-I) comprising coxa, basis and three-segmented rami; intercoxal sclerite well developed; basis of P1 and P3 with naked outer seta and round process between insertions of enp and exp, and exopods distinctly longer than endopods.
Exopods of P1 to P3: inner margin of first and second segments with long setules, first segments of P1 to P3 without spine, and relative length ratio of terminal spine to distal outer spine of P1 to P3 different: P1 smallest (2.2 : 1), P2 (2.6 : 1), and P3 largest (3.2 : 1), terminal spines longer than distal segments, in P1 1.2 times longer, in P2 about 1.5 times longer, and in P3 2.3 times longer.
P4 (Fig. 3J) with narrow, transversely extended, intercoxal sclerite; coxa unarmed; basis with outer basal seta arising from posterior surface, fringed with row of setules along inner margin; exp well developed, three-segmented, bearing spinules along inner margin of first segment, proportional length ratio of each segment 37 : 18.5 : 44.5, distal segment 1.8 times as long as terminal spine; enp absent. Armature formula of P1 to P4 as follows (Roman numerals indicate spines, and Arabic ones, setae):
P5 (Fig. 2B) consisting of two unequal simple setae located ventrolaterally.
P6 (Fig. 2A, B) represented by operculum closing off each genital aperture (arrowed in Fig. 2B).
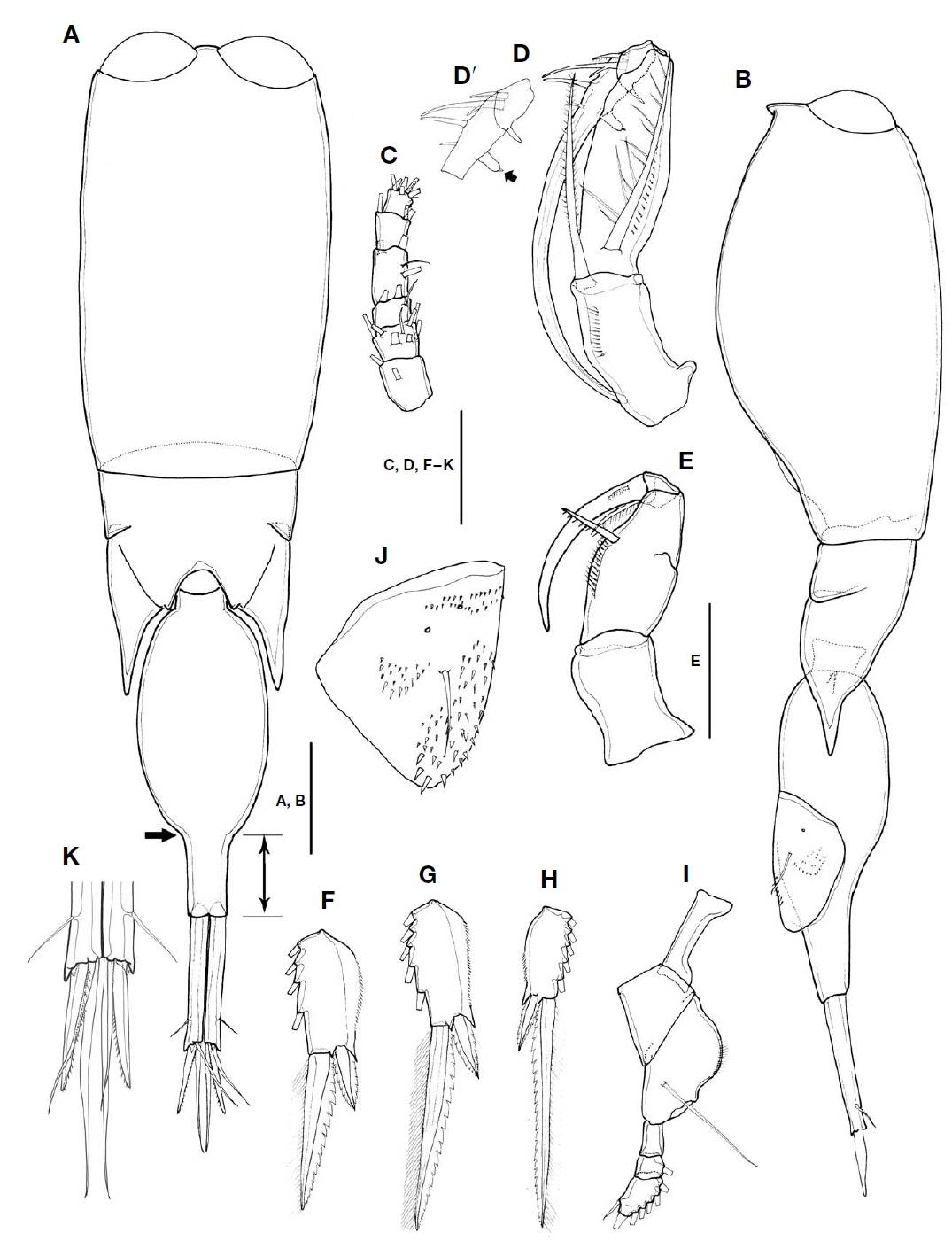
Male. Total body length in lateral view 958-986 μm(n=3). Urosome distinctly narrower than prosome (Fig. 4A, B).
Prosome two-segmented, two large contiguous cuticular lenses located at a distance from each other on frontal part: prosomal length about 1.2 times as long as urosome including caudal rami, 1.6 times as long as urosome length excluding CR (Fig. 4A, B); fourth pedigerous somite with extended and pointed posterolateral corners, extended to 26% of second urosomal somite (Fig. 4A, B).
Second urosomal somite (Fig. 4A, B): anterior margin rounded, 2.5 times longer than maximum width (at anterior two-fifths), posterior part abruptly narrowed (indicated by arrows in Fig. 4A), and rear margin about 0.32 times as long as rest of second urosomal somite (marked in Fig. 4A), and 1.5 times longer than caudal ramus.
CR 6.8 times longer than width at base (Fig. 4A, K), about 0.38 times as long as second urosomal somite. Armature of rami similar to that of female. Dorsal seta V slightly longer than seta III.
Antennule (Fig. 4C) short, 6-segmented; armature formula as in female; proportional lengths (%) 23 : 14.7 : 11.5 : 24.6 : 16.4 : 9.8.
Antenna (Fig. 4D, D′) sexually dimorphic, four-segmented, with coxa and basis fused and endopod three-segmented; coxobasis 1.6 times longer than width at base, with long bipinnate strong seta on inner distal margin, slightly shorter than length of first endopodal segment, fringed with row of spinules along inner margin; first endopodal segment about 3 times as long as width at base, bearing bipinnate seta on ventral proximal margin, almost as long as coxobasal seta, outer lateral margin ornamented with row of denticles; second endopodal segment short, bearing three elements: stout spine arising from outer distal margin, with lateral branch, short plumose spine located near stout spine, and short blunt spine arising from inner distal margin; third endopodal segment drawn out into long claw, armed with four elements: short spine arising from proximal inner margin, tipped with minute process (arrowed in Fig. 4D′); long naked seta and two slender, naked setae inserted on outer proximal margin.
Mandible, maxillule, and maxilla similar to those of female.
Maxilliped (Fig. 4E) sexually dimorphic, four-segmented, comprising syncoxa, basis and two-segmented endopod (subchela); syncoxa without surface ornamentation, unarmed; basis robust, oval, particularly swollen in proximal half, inner margin with spiniform seta ornamented with several tough spinules along inner margin, with row of fine spinules between proximal third and seta, twice as long as width at base, and 2.3 times longer than sea on basis. Subchela comprising unarmed proximal endopodal segment and distal endopodal segment drawn out into long curved claw, with accessory armature consisted of minute, unipinnate spine on outer proximal margin, and long unipinnate spine delimited basally from inner proximal corner of claw.
P1-3 (Fig. 4F-H) segmentation and armature similar to those of female, except for length ratios of terminal spine to distal outer spine of P1 (1.3 : 1), P2 (3 : 1), and P3 (3.9 : 1).
P4 (Fig. 4I) similar to that of female, except proportional length ratio of each segment 37.7 : 23.1 : 39.2, and length ratio of distal segment to terminal spine smaller (1.5 : 1) than that of female (1.8 : 1).
P5 (Fig. 4B) similar to that of female.
P6 (Fig. 4B, J) represented by genital flap closing off each genital aperture, armed with long seta, surface ornamented with unique pattern of denticles and two small secretory pores: inner part covered with small denticles to anterior third from distal margin, outer margin ornamented with denticles arranged in semicircle in mid region; distal margin with comparatively large denticles.
Etymology. The species named in honor of Dr. M. Dahl (Germany), in recognition of her many contributions to the taxonomy of the Corycaeidae.
Remarks. Farran (1911) eastablished a new genus
Most taxonomic records of
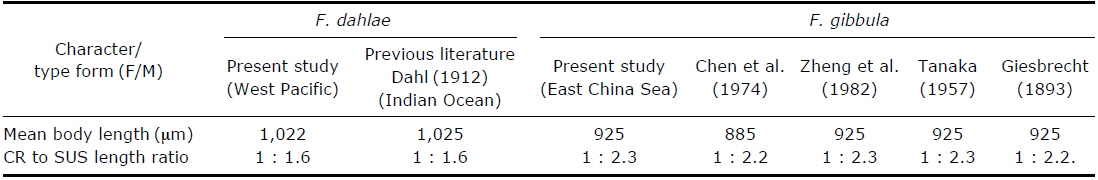
[Table 1.] Morphological differences between Farranula dahlae n. sp. and F. gibbula
Morphological differences between Farranula dahlae n. sp. and F. gibbula
In this study, we discovered that the entire surface of the caudal rami of females of both species are ornamented with small denticles and the distal margin has a unique shape: the distal margin of the caudal rami of
The similar morphologies of the second urosomal somite of
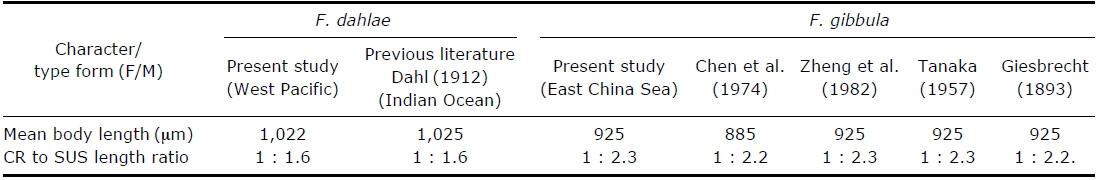
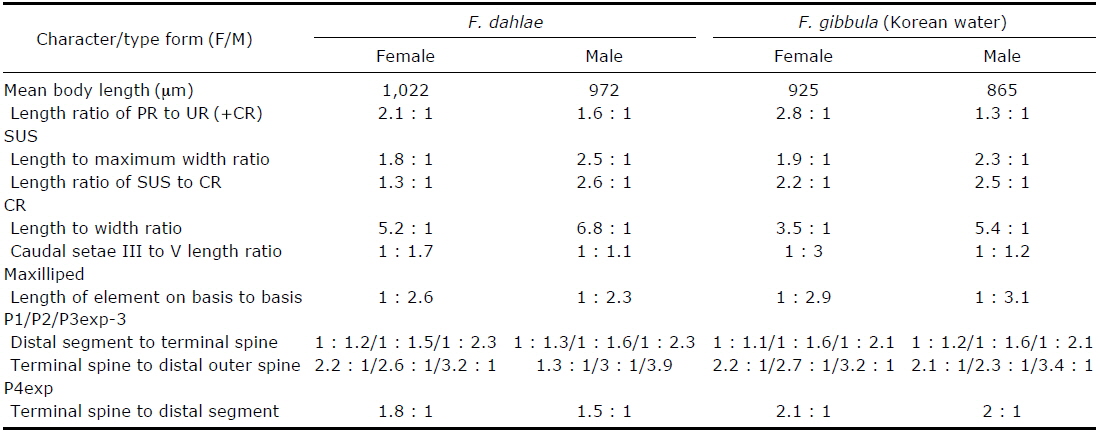
A comparison of morphological characteristics of Farranula dahlae n. sp. and F. gibbula in published literature.
In order to test for geographical variation, females of