Porcine circovirus type 1 and type 2 (PCV1 and PCV2) are very small (about 17 nm), spherical, non-enveloped viruses with icosahedral symmetry (Allan and Ellis, 2000). PCVs have circular, single-stranded DNA genomes of approximately 1700 nucleotides, encoding two major open reading frames, ORF1 and ORF2. ORF1 is predicted to encode a replicase protein that is essential for replication of the viral DNA (Nawagitgul
Since the mid 1980s, insect baculoviruses have been utilized as expression vectors of foreign genes (Choi
Previously, we reported that the BEVS is sufficient to production of PCV2 capsid protein (Lee
The
>
Construction of transfer vector
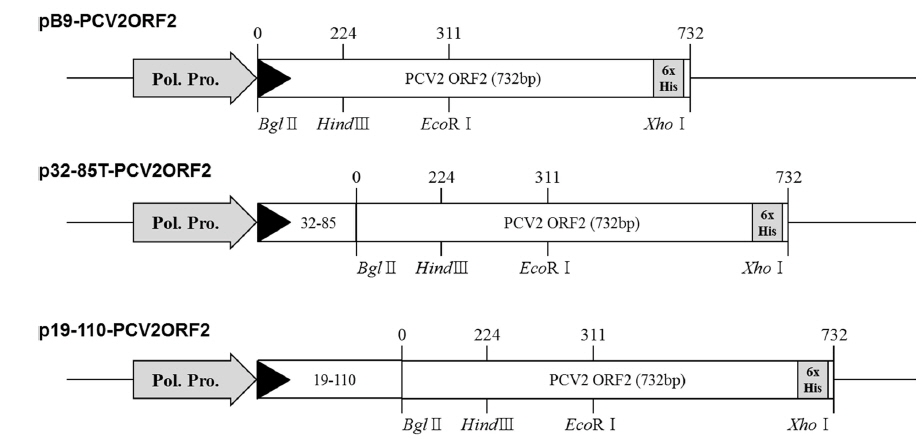
The previously constructed pB9-PCV2ORF2 (Lee
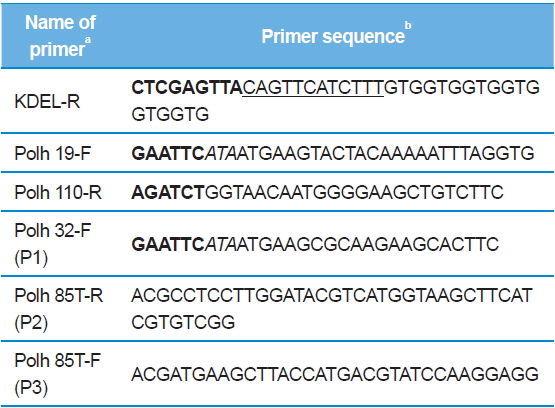
[Table 1.] Primers used for amplification and sequencing in this study
Primers used for amplification and sequencing in this study
>
Generation of recombinant baculoviruses
Recombinant AcMNPVs expressing various encoded fragments under the control of the polyhedrin promoter were generated by co-transfection with each transfer plasmid and a defective viral genome, bAcGOZA DNA (Je
>
SDS-PAGE and Western blot analysis
The cell lysate was prepared by incubating the cells with RIPA buffer (20 mM Tris-HCl pH 7.5, 50 mM NaCl, 5% glycerol, 0.1% Triton X-100) containing a protease inhibitor cocktail (Sigma-Aldrich, USA) for 30 min on ice followed by sonication; then, the lysate was mixed with protein sample buffer and boiled. The protein samples were subjected to 12% SDS-PAGE and then transferred to a PVDF membrane. The membranes were blocked in 5% milk in Tris-buffered saline containing 0.05% Tween 20 and probed with each of the following antibodies: His-tag monoclonal antibody (Cell Signaling, USA) and PCV2-ORF2 monoclonal antibody (JENO Biotech, Korea). The membrane was then incubated with horseradish peroxidase-coupled anti-mouse IgG antibody (Cell Signaling, USA), and the bound antibodies were detected using an enhanced chemiluminescence system (Merck Millipore, Germany) according to the manufacturer’s instructions.
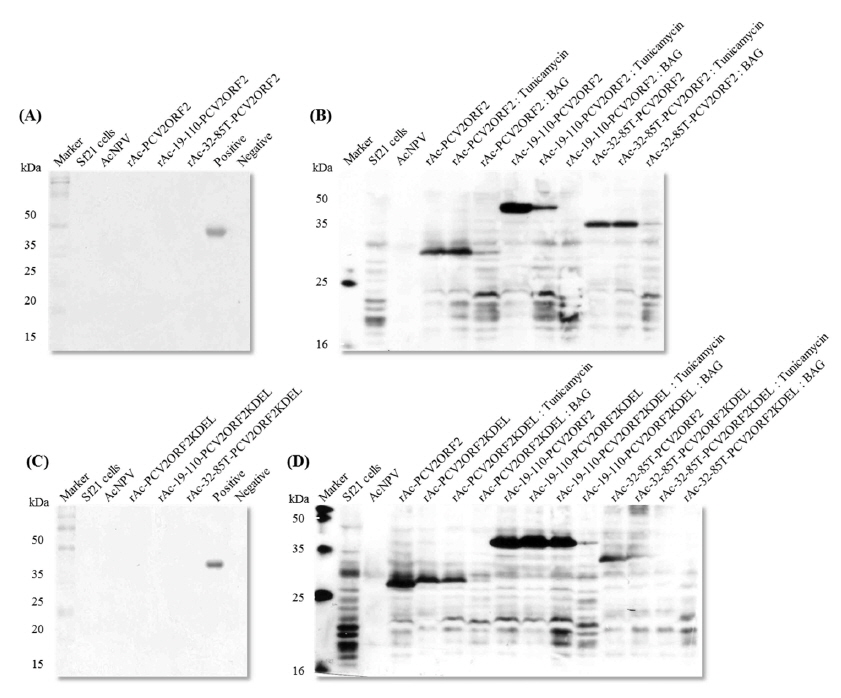
Glycosylation of the recombinant protein was determined using Gelcode Glycoprotein Staining Kit (Pierce Biotechnology, USA). For linked site confirmation, the infected medium was replaced with fresh serum-free insect cell culture medium including 5 μg/mL tunicamycin or a final concentration of 3 mM Benzyl-N-acetyl-α-d-galactosaminide (BAG). The harvested cells were immunoblotted by the methods previously described for protein analysis using SDS-PAGE and Western blot analysis.
The rAc-PCV2ORF2, rAc-19-110-PCV2ORF2, and rAc-32-110-PCV2ORF2 protein samples were subjected to 12% SDS-PAGE. After running the gel, a strip 7 mm in width, encompassing proteins of the apparent molecular weights of 28, 40, 35 kDa were excised and proteins were extracted using the EzWay PAG Protein Elution Kit (KOMAbio-tech, Korea). Each eluted protein was diluted in coating buffer (3.7 g bisodium carbonate, 0.64 g sodium carbonate, pH 9.6) at ratios of 1:4, 1:2 and 1:1, respectively. After 3 h of incubation at 4 ℃, plates were washed three times with TBS-T (6.06g Tris Base, 8.7g NaCl, pH 7.2, 0.05% Tween 20), blocked for 2 h at 37 ℃ with a blocking solution containing TBS-T and 2% bovine serum albumin (BSA), and then incubated with anti-PCV2 ORF2 monoclonal antibody. After washing three times with TBS-T, the plates were incubated with 100 μL of diluted secondary antibody, anti-mouse-IgG antibodies conjugated to horseradish peroxidase (Cell Signaling, USA), for 2 h at 37 ℃. Reaction products were washed with TBS-T and 50 μL of tetramethylbenzidine (TMB) (Sigma-Aldrich, USA) was added to each well. After incubation for 15 min at room temperature in the dark, the reaction was stopped by adding 50 μL of stop solution. The absorbance of each well was measured with a spectrophotometer at 450 nm.
>
Enhanced production of recombinant capsid protein
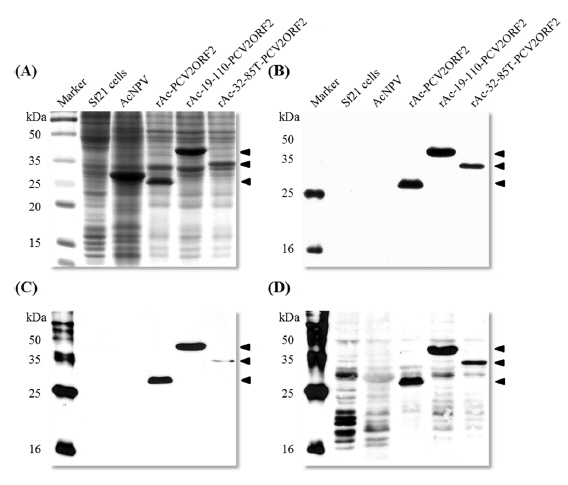
Recombinant viruses were generated to express partial polyhedrin fused to the N-terminus of the PCV2 ORF2 under the control of the AcMNPV polyhedrin promoter (Fig. 1). To analyze the expression of the recombinant protein, Sf21 cells were infected with recombinant viruses and harvested at 4 d post infection. Recombinant capsid proteins were detected at approximately 28 kDa, 40 kDa, and 35 kDa by rAc-PCV2ORF2, rAc-19-110-PCV2ORF2, and rAc-32-85T-PCV2ORF2, respectively (Fig. 2). These sizes corresponded to the predicted protein sizes with the fused partial polyhedrin. However, cleavage of the recombinant protein when fused with polyhedrin 32–85 was not observed. This result suggested that the effect of partial polyhedrin fusion to the target protein may vary according to the target protein (Bae
As the yield of all recombinant capsid proteins was very high, the production of each recombinant protein was compared to the others with the same conditions of infection (Fig. 4). Fusion expression with partial polyhedrin 19–110 increased the production of recombinant capsid protein, but fusion with 32–85 could not increase production above that of the non-fused recombinant protein. Also, recombinant capsid proteins were detected with anti-PCV2 ORF2 monoclonal antibody or anti-PCV2 porcine serum, as well as anti-His monoclonal antibody. Although fusion of polyhedrin 19–110 increased the yield of recombinant PCV2 ORF2, it was lower than that of enhanced green fluorescent protein (EGFP) (Bae
>
Glycosylation and immunogenicity analysis
In our previous report, we did not observe the glycosylation of recombinant PCV2 capsid proteins in insect cells (Lee
The immunogenicity of recombinant capsid proteins as diagnostic antigens was analyzed by an ELISA method with direct coating of antigens. The antigen levels were inversely proportional to the antigen dilution (Fig. 6). These levels were highest using the recombinant capsid protein fused with polyhedrin 19–110. This result suggested that partial polyhedrin fusion expression of PCV2 ORF2 can enhance not only the yield of capsid protein but also its immunogenicity.