Zophobas atratus Fabricius (Coleoptera: Tenebrionidae) is a large neotropical beetle. It is found on fruit bat guano and organic litter in its natural environment (Tschinkel and Willson, 1971; Quennedey et al., 1995). The shape of this beetle is similar to that of mealworm, Tenebrio molitor, a well-known food resource for small pets, but it is several times larger than a mealworm. It is not a domestic species of Korea, but originated from South and Central America (Park et al., 2013). In the Animal and Plant Quarantine Agency notification 2013-118, this beetle has been included in the list of excluded quarantine pests in 2011 , and has been allowed to be imported live as animal feed (Animal and Plant Quarantine Agency, 2013).
Z. atratus is larger than T. molitor, and is also known as super worm or king worm (Park et al., 2013). It has been used as a protein source for small pets such as birds, reptiles, and small mammals (Jabir et al., 2012; Park et al., 2013). Moreover, because of the rising prices of fish meal, which is widely used a protein source in animal feed, alternative protein sources are sought to reduce the production cost of various animal feeds. Recently, insect-based diet has become a strong candidate as an alternative to fish meal because fish raised on insect-based diet showed similar growth performance to those raised on conventional fish meal-based diet (Jabir et al., 2012).
Much attention has been focused on Z. atratus because of its economic benefits. Previous studies have reported that Z. atratus is highly dependent on isolation for the onset of metamorphosis (Quennedey et al., 1995; Aribi et al., 1997). In crowded conditions, Z. atratus larvae showed increasing size and weight, forming supernumerary larval instars that did not pupate until death (Tschinkel and Willson, 1971). This inhibition of pupation induced by crowding conditions has also been described in other tenebrionid species, for example, Tribolium freeman (Nakakita, 1982; Kotake et al., 1993; Quennedey et al., 1995).
The number of post-embryonic molts in T. molitor is known to range from eight to more than 20 larval molts, depending on various intrinsic and extrinsic factors, such as food quality and individual density (Connat et al., 1991). Previous studies have focused on various abiotic factors, including food quality, humidity (Murray, 1968; Urs and Hopkins, 1973), temperature (Ludwig, 1956), and photoperiod (Tyschchenko and Sheyk Ba, 1986) that affected the number of larval instars. However, the characteristics of different larval stages of Z. atratus commercialized in Korea remain unclear. In addition, the precise number of instars that Z. atratus larvae go through at the optimal temperature of 25℃ is almost unknown. To this end, we investigated the characteristics of Z. atratus during different larval stages and determined the average number of larval instars. The results of this study will provide basic information for future investigations on the physiological characteristics of different instars, as well as for more effective commercialization of Z. atratus.
Z. atratus adults (about 120 individuals) were raised in acrylic boxes (58.2 cm width × 49.5 cm length × 17 cm height) at 25±3℃, 50–70% relative humidity (RH) and a photoperiod of 14L:10D. In each acrylic box, wheat bran (ca. 3 cm deep) was placed as a food source, and a Chinese cabbage leaf was placed on the wheat bran layer as a moisture source. Once a mating couple was observed, they were moved to a petri dish (10 cm diameter × 1 cm height) containing bran. Eggs with hardened shells were obtained after three d. We collected 40 eggs to count the number of larval instar and to measure the body size. Each egg was transferred to a petri dish (5 cm diameter × 1.5 cm height) containing wheat bran and Chinese cabbage (1 g). This experiment was repeated 3 times.
Each egg was reared in a petri dish (5 cm diameter × 1.5 cm height) with wheat bran at 25±3℃, 50–70% RH, and 14L:10D photoperiod. The condition of the larvae was checked every day to determine the number of instars. When the larval exuvium was observed, the larvae were removed from the petri dishes. After pupation, instar counting was stopped.
To measure the body length of each instar, the body lengths of ten larvae were measured using a Vernier caliper after each larval molting. Generally, the width of the head capsule was measured because it exhibits distinct variation between the larval stages (Hsia and Kao, 1987). In this experiment, however, the body lengths of larvae were also measured because of the small head capsule size during the early larval stages. In addition, we took photographs of each instar using a digital camera (SX220 HS, Canon, Tokyo, Japan ).
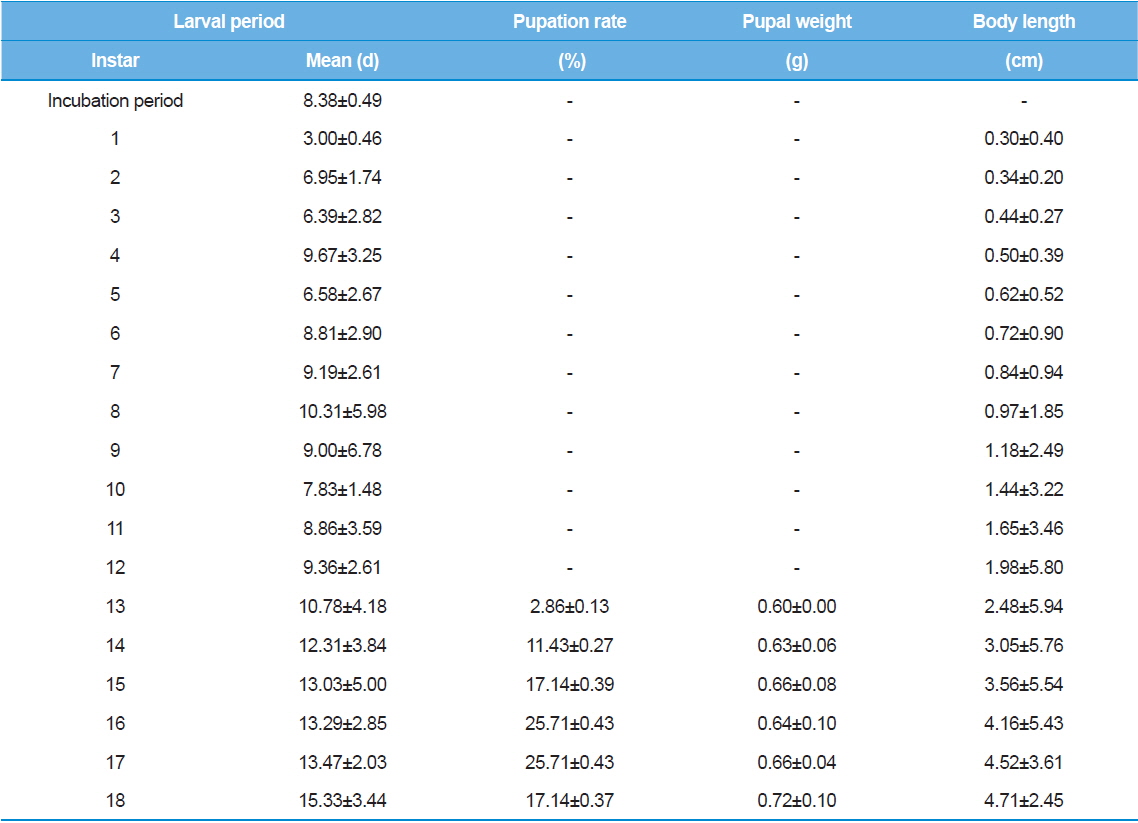
As shown in Table 1, the average incubation period for hatching was eight – nine d, and the duration for the first instar three or four d. Interestingly, the size of larvae was not significantly different for different incubation periods or first instar durations. Between the second and 18th instars, the duration of each instar showed large variations among individuals. The first pupation was observed at the 13th instar even though only 2% of larvae were pupated.
Previous studies have shown that the body size of larvae is highly co-related with the pupation rate (Sehnal, 1985). The developmental variability in tenebrionid beetle depends on two parameters: the number of larval instars and the duration of each larval instar. Previous studies have suggested that juvenile hormones play a key role in the mealworm developmental variability; the alimentary periods as well as the percentage of larval molts are increased by juvenoids (Feyereisen, 1985; Connat et al., 1991). In addition, Z. atratus is a model organism to study the relationship between external events and endocrine factors that regulate the onset of insect metamorphosis (Riddiford, 1976).
The lack of uniformity among larvae between the second and 18th instars may be caused by variations in nutrition. A study on Manduca sexta by Nijhout (1975) revealed that the number of instars increased under poor nutritional conditions. However, it remains to be verified whether this phenomenon occurs in Z. atratus or not. Furthermore, the symptoms caused by pathogens are highly similar to those of poor nutritional conditions in Z. atratus. It was also revealed that the gregarious nature of T. molitor enhanced its ability to resist pathogens (Barnes and Siva- Jothy, 2000). Therefore, further studies are required to investigate whether the duration of each instar is influenced by nutritional status, pathogen activity, or larval behavior.
The first pupation was observed at 13th instar. Approximately 85.70% of pupation was observed between the 15th and 18th instars. The largest proportion of pupae (25.71%) was observed at the 16th and 17th instars. In other words, most of the larvae in this experiment exhibited 15 to 18 instars in their life cycle. T. molitor larvae from old parents showed shorter larval period (Tracey, 1958). Moreover, the growth rate of offspring from old parents was delayed (Fiore, 1960). Therefore, we suggest that further studies should investigate larvae from the same age groups to reduce the effects caused by the parents’ age. Further studies using a wide range of rearing temperatures might elucidate the effects of rearing temperature on the number of larval stages.
Body length of Z. atratus instars increased gradually, and reached the maximum body length in the 18th instar. Mortality was mostly observed between the second and fifth instars; one larva died in the 17th instar. Based on these results, we conclude that more intensive care during the early stages of Z. atratus larvae leads to successful pupation when the larvae are reared individually.
The first instar was white, and gradually turned brown from or after the second instar. In particular, the anterior and posterior ends were darker than the middle of the body. Except for the color change, no significant morphological differences were detected in Z. atratus larvae (Fig. 1). We also checked the cuticle color, which typically changes based on the population density (Applebaum and Heifetz, 1999), and has been correlated with resistance in a lepidopteran (Reeson et al., 1998).
We observed more severe cannibalism in pupae or larvae that were close to molt in Z. atratus under grouped conditions. In addition, successful pupation requires enough space for the each last instar larva. Previous study showed that successful pupation was affected by population density because only Z. atratus larvae with enough isolated space successfully pupated (Tschinkel, 1981). Cannibalism of pharate pupae by active larvae may be the selective factor that evolved to the inhibition of pupation by crowding, higher larval growth rates, and higher fecundity (Tschinkel, 1978; Tschinkel, 1981).
In this study, we determined the incubation period of eggs, the duration of the first instar, and the average number of larval instars in Z. atratus. Our results will provide a basis for further studies on this species, as well an insight into an important protein source in commercial feeds for various animals.