Red lip mullet Chelon haematocheilus (body weight = 468 ± 91 g) which became sick during an outbreak of disease at mariculture facilities at Cheonsu Bay, Korea, during July–August 2013, were examined to identify the cause of the disease. Diseased mullets displayed green liver syndrome, and Lactococcus garvieae were isolated from their internal organs. Argulus sp., Trichodina sp., and/or Vibrio spp. were also discovered in some infected fish. Histopathological examination revealed that fatty liver syndrome with hepatocyte degeneration, reflected in heterokaryons, inflammatory lesions, and melanomacrophage centers (MMCS), had caused fibrosis around the kidney, spleen, and blood vessels. After the outbreak, visceral fat and green liver syndrome in the mullets were consistently observed throughout the year in the same mariculture facilities, indicating that the cultured mullets suffered a chronic metabolic disorder. Although Vibrio spp. were also isolated from some individuals, L. garvieae, which is known to be a causative agent of red lip mullet mortality, was isolated from all diseased individuals. This is the first report of L. garvieae infection in cultured red lip mullet.
Coastal mullets are the most widely distributed of the bony fishes in both the temperate and tropical regions of the Pacific and Atlantic oceans. Grey mullet
Green liver syndrome refers to macroscopic observations of fish liver having a dark- or light-green color rather than its normal light brown appearance. Green liver syndrome is frequently observed in red sea bream
During July–August 2013, mortalities were noted in cultured red lip mullet stocks in Cheonsu Bay on the west coast of Korea. The dead fish displayed abdominal distension and green liver syndrome. We undertook an investigation to identify the cause of the disease.
>
Disease outbreak and fish sampling
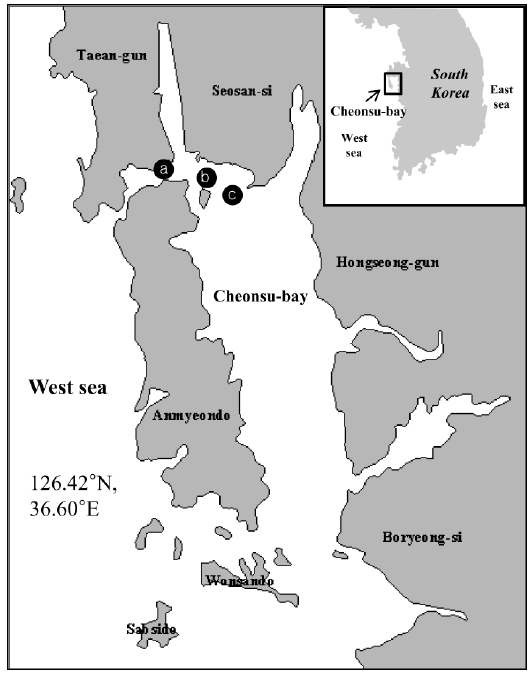
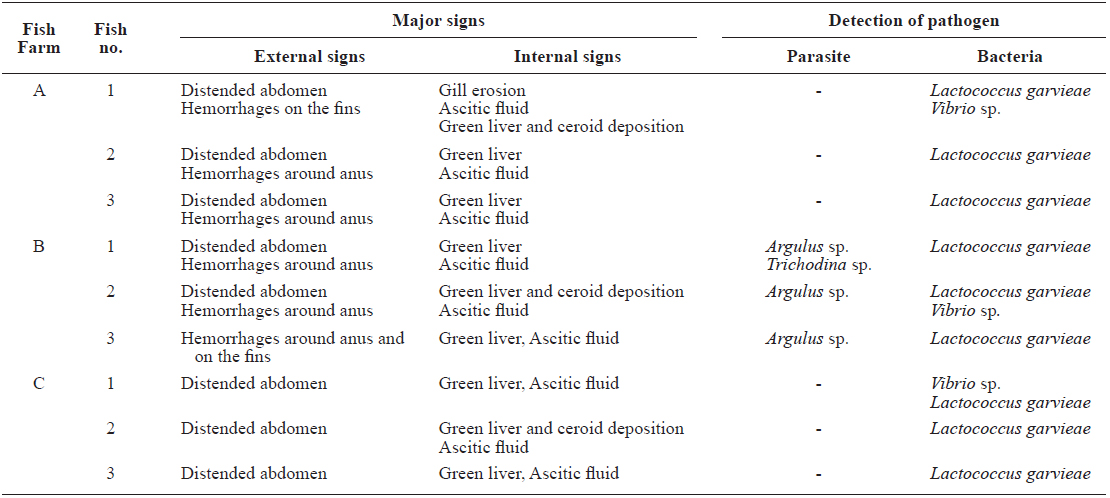
Mass mortality of red lip mullets displaying abdominal distension and green liver syndrome, was recorded at three fish farms (Fig. 1; Farms A, B and C) in Cheonsu Bay, Chungcheongnam-do, from late July until mid-August 2013. At farms A and C, where approximately 100,000 red lip mullet were cultivated, an average of 100–150 fish died over 15 consecutive days. The cumulative mortality accounted for 2.1% of the total fish stock (average fish body weight farm A = 384 ± 109 g, farm C = 498 ± 70 g). At farm B, where approximately 100,000 red lip mullet were cultivated, an average of 200–250 fish died over 15 consecutive days. The cumulative mortality accounted for 3.5% of the total fish stock (average fish body weight 523 ± 10 g). Three moribund red lip mullet from each farm were used in this study.
>
Parasite and virus examination
Parasite infection was examined using a speculum to obtain biopsy samples comprising the gills, body surface, fin slime, and internal organs (brain, kidney, and spleen) of red lip mullet. Marine birnavirus (MBV; MBV-F: 5′-GCACCACGAAGGTACGAAAT-3′, MBV-R: 5′-GTACGTTGCCGTTTCCTGAT-3′), viral hemorrhagic septicemia virus (VHSV; VHSV-F: F: 5′-ATGGAAGGAGGAATTCGTGAAGCG-3′, VHSV-R: R: 5′-GCGGTGAAGTGCTGCAGTTCC-C-3′), hirame rhabdovirus (HRV; HRV-F: F: 5′-TGAGGTAGAGCAAGCCAACA-3′, HRV-R: R: 5′-ACATGACTGGCCGAAAAGAG-3′), viral nervous necrosis virus (VNNV; VNNV-F: F: 5′-CGTGTCAGTCATGTGTCGCT-3′, VNNV-R: R: 5′-CGAGTCAACACGGGTGAAGA-3′), and red seabream iridovirus (RSIV; RSIV-F: F: 5′-CTCAAACACTCTGGCTCATC-3′, RSIV-R: R: 5′-GCACCAACACATCTCCTATC-3′) were detected by polymerase chain reaction (PCR). Internal organs including the kidney and spleen were removed from each fish. DNA for PCR was isolated using a High Pure PCR Template Preparation Kit (Roche, Basel, Switzerland), RNA was isolated using TRIZOL® Reagent (Invitrogen, Carlsbad, CA, USA) according to the manual, and cDNA was produced using SuperScriptTMII Reverse Transcriptase (Invitrogen, USA). The isolated DNA was applied as a template for the analysis of RSIV PCR, and the synthesized cDNA was used for the analysis of VHSV, VNNV, HRV, and MBV PCR. PCR was conducted using ExTaq® (Takara, Shiga, Japan). The amplified products were verified by 1.5% agarose gel electrophoresis (Bioneer, Daejeon, Korea), and all PCR analyses were compared through positive and negative contrasts.
The liver, kidney, and spleen of moribund fish were inoculated onto brain heart infusion agar (BHIA; Difco, Sparks, MD, USA) containing 1% (w/v) sodium chloride (BNA), and incubated at 25°C for 24-48 hours. Hemolytic activity was determined by 5% sheep blood agar medium (Micro Media Co., Ltd, Seoul, Korea) at 25°C for 48 hours.
(a) Biochemical analysis
All strains were tested for biochemical characteristics using the API 20E and API strep systems (BioMerieux, Marcy l’Etoile, France) according to the manufacturer’s protocols. The oxidase test consisted of a cytochrome oxidase paper test and catalase slide test using 3% H2O2.
(b) 16S rRNA gene sequencing
Bacterial genomic DNA was isolated using the High Pure PCR Template Preparation Kit and ExTaq® following the manufacturer’s protocol. The DNA templates were amplified using PCR in a Gene Amp PCR System 2400 (Perkin Elmer; Wellesley, MA, USA) using universal primers amplifying a 1343-bp region of the 16S rRNA gene (63F: 5′-CAGGCCTAACACATGCAAGTC-3′; 1406R: 5′-ACGGGCGGTGTGTRC-3′) obtained from Bioneer. The DNA templates were amplified by initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extending at 72°C for 1.5 min, and a final extension at 72°C for 5 min. Direct sequencing of the amplified DNA fragment was performed using a 3730xl DNA automatic sequencer (Applied Biosystems; Foster City, CA, USA). All sequences were submitted for similarity searches with BLAST (Altschul et al., 1990).
The tissues collected from each individual were fixed in 10% neutral buffered formalin (NBF). After fixation, standard histological procedures were used for tissue dehydration and paraffin embedding. Tissue sections were stained with hematoxylin–eosin (H&E), and the tissue specimens were observed under an optical microscope (Zeiss, Jena, Germany).
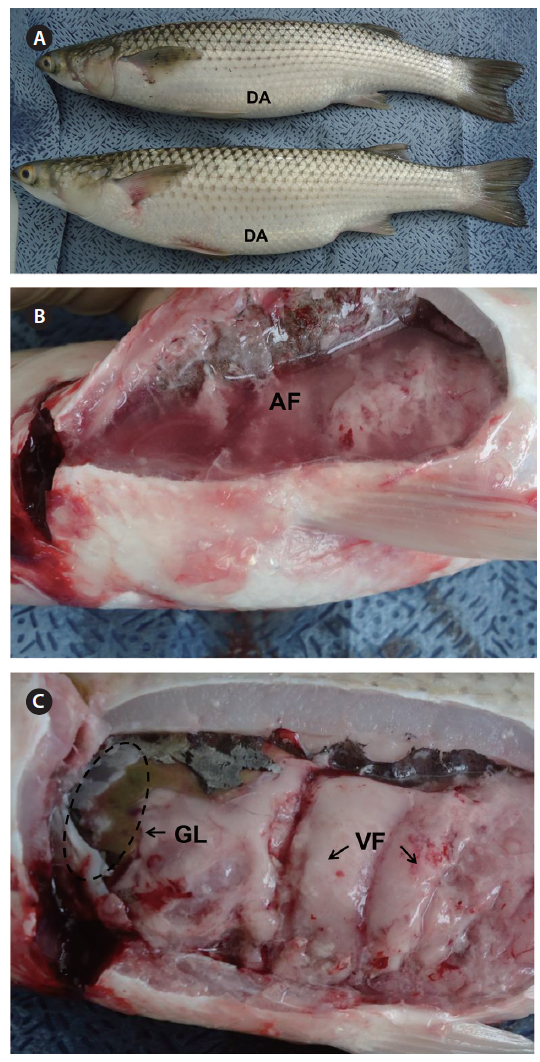
All red lip mullet investigated at the three fish farms displayed abdominal distension, and some had petechial hemorrhages and congestion or hemorrhages at the base of the fins as external symptoms (Fig. 2A). Ascites and green liver syndrome appeared as internal symptoms (Fig. 2B, 2C).
>
Parasite and virus examination
[Table 1.] Diagnostic results of this study
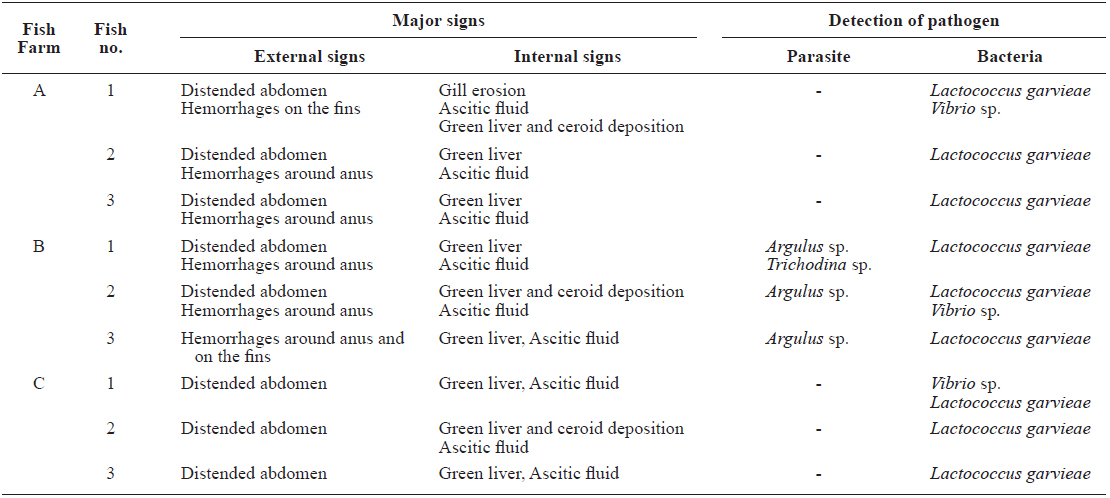
Diagnostic results of this study
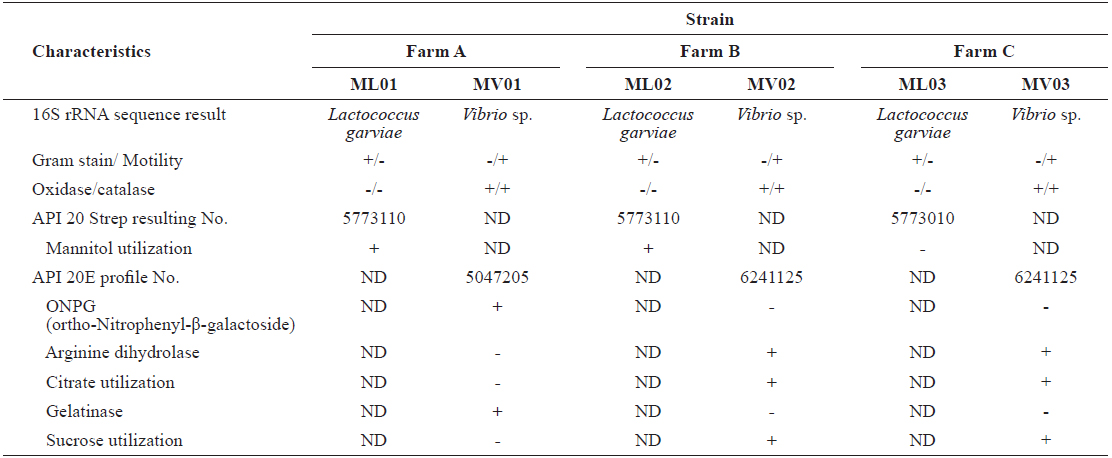
The prevailing milky white colonies (ML-01, ML-02, and ML-03) were grown on BNA smeared with kidney and spleen samples from all fish (
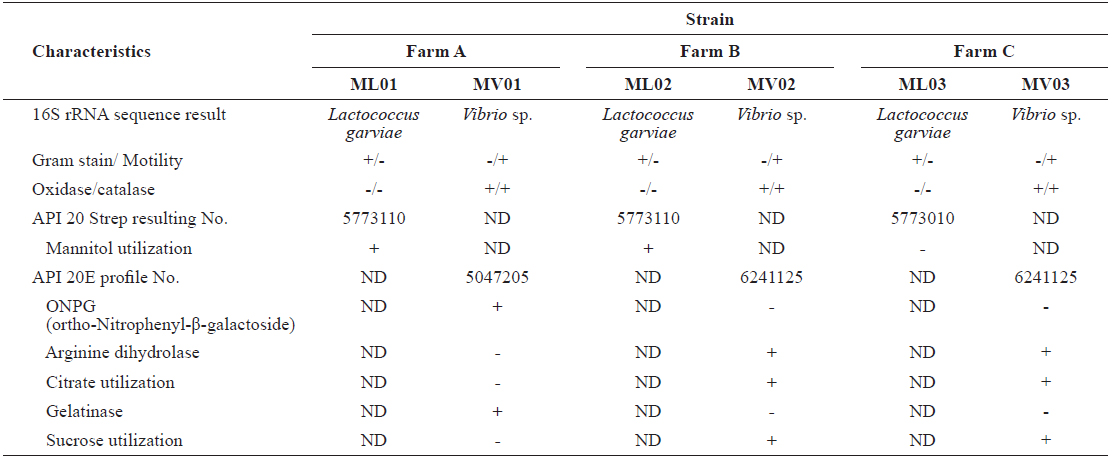
Characteristics of Lactococcus garvieae and Vibrio spp. strains isolated from red lip mullet Chelon haematocheilus
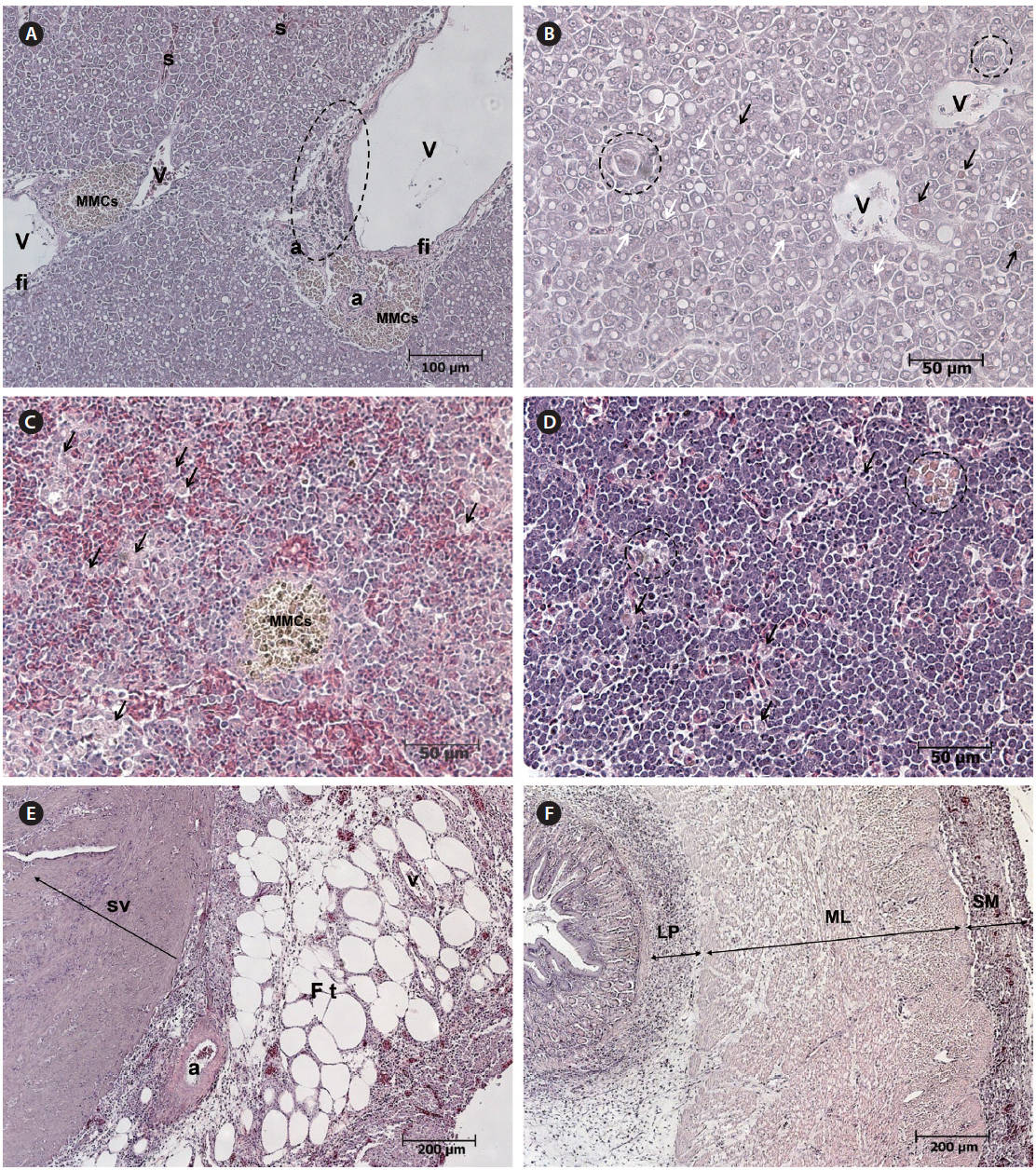
Histopathological examination revealed apparent fatty liver changes, with hepatocyte degeneration apparent as heterokaryons or fissile materials and the cytoplasm of degenerated cells containing cell debris. The number and size of fat droplets (i.e., white vacuoles) varied, and the size, shape, and arrangement of hepatocytes were irregular (Fig. 3A, 3B). Degenerated cells were surrounded by epithelioid cells, which is the initial stage of tuberculation (Fig. 3B). Inflammatory degeneration, melanomacrophage centers (MMCS), and fibrosis were identified around blood vessels (Fig. 3A). MMCs in various sizes and an increase in degenerated macrophages containing phagocytosed substances were observed in the spleen (Fig. 3C). An aggregation of macrophages with brown pigmentation was occasionally observed in the head kidney (Fig. 3D), and weak eosinophilic degeneration was sometimes noticed in the epithelium of renal tubules (data not shown). Inflammatory cell infiltration was observed in serous membranes and fat tissue around the heart and in the connective tissue layer of the digestive tract (Fig. 3E, 3F).
Although
All red lip mullet specimens showed visceral fat with green liver syndrome as internal symptoms, regardless of the fish farm investigated (Fig. 2C). The visceral fat and green liver syndrome were observed consistently throughout year at all three farms (data not shown). A fat metabolism problem was also identified, as the distribution of fat cells in the liver was histopathologically irregular. The greenish-brown liver tissue likely had local cholestasis, with a fat metabolite such as lipofucsin producing a bile duct obstruction. In previous studies, green liver syndrome in grey mullet cultivated in Korea was observed to persist throughout the year (Lee, 2008), as was also found in this study. Green liver syndrome in grey mullet is related to nutrition hypertrophy in hepatocytes due to excessive nutrient absorption, suggesting that feeding suspension might improve green liver syndrome (Lee, 2008). The cause of green liver syndrome in the diseased mullets was estimated to be hypernutrition, with the use of low quality assorted feeds that are currently available in Korea also having a negative influence on mullet health. A nutritional imbalance may be created when the feedstuff lacks essential proteins such as taurine. The mullet feed available in the market in Korea is cheaper than the assorted feed used for other seawater fishes, which consists of a low-fishmeal diet (less than 30%) and high soy protein concentrate (Jeil Feed Co., Ltd, personal communication). Fishmeal is a finite resource, with limited availability and high demand, and it is the most expensive protein ingredient in fish diets. It is necessary to investigate any correlations between feed ingredients, the amount provided, and the incidence of disease, as the feedstuffs provided at all three of the fish farms where disease occurred were available in the local Korean market.
It is also likely that the mass mortalities of red lip mullet in Cheonsu Bay were related to changes in water temperature. The temperature appeared to rise sharply from 22°C in mid-July to 26-28°C in late July through early August (http;//egov.nfrdi.re.kr-Comprehensive Status Information). This period of rising water temperature is consistent with the mass mortality period.