In an effort to discover alternative phytotherapeutic antimicrobial agents to combat Streptococcus parauberis, a fish pathogenic bacterium, we evaluated the antibacterial activity of seaweed extracts in vitro. A methanolic extract of Ecklonia cava exhibited strong antibacterial activity against S. parauberis isolated from olive flounder Paralichthys olivaceus. Furthermore, the n-hexane soluble (Hexane) fraction of the E. cava methanolic extract exhibited the greatest antibacterial effect on S. parauberis strains with a minimum inhibitory concentration (MIC) ranging from 256 to 1,024 μg/mL. In addition, the MIC values of oxytetracycline against antibiotic-resistant S. parauberis were markedly reduced up to 64-fold in combination with the Hexane fraction, suggesting that the antibacterial activity of the antibiotic was restored when combined with the Hexane fraction. The interaction between both antibiotics and the Hexane fraction was assessed by the fractional inhibitory concentration (FIC) index. The Hexane fraction and oxytetracycline combination against antibiotic-resistant S. parauberis strains resulted in a median ∑FIC range of 0.502 to 0.516. Thus, the synergistic ranges of median ∑FIC < 1 were observed for all combinations of the Hexane fraction and oxytetracycline against S. parauberis. To the best of our knowledge, this is the first report indicating the efficacy of an E. cava extract against fish pathogenic bacterium S. parauberis.
Over the past several years, streptococcal infections have dramatically increased due to the growth of aquaculture (Baeck et al., 2006; Park et al., 2009). Streptococcosis is a common disease in fish caused by six different Gram-positive streptococcal species (Bercovier et al., 1997). One species,
Broad-spectrum antibiotics are commonly used to control streptococcal infections in aquaculture. Park et al. (2009) reported the isolation of antibiotic-resistant
Seaweeds contain various metabolites that are capable of inhibiting bacterial growth (Rhimo et al., 2010; Cox et al., 2010; Eom et al., 2013). Among these seaweed species, edible brown alga
>
Bacterial strains and growth conditions
In this study, we used
>
Samples and extraction method
In total, we used thirteen seaweed species. Dried seaweed powder (1.0 kg) was extracted and fractionated using organic solvents as described by Lee et al. (2014). The methanolic (MeOH) extract of
We evaluated the antibacterial activity of seaweed extracts and antibiotics against
The sensitivity of the
>
Checkerboard method using a fractional inhibitory concentration (FIC) assay
Interactions between the
>
Antibacterial activity of MeOH extracts from thirteen marine algae
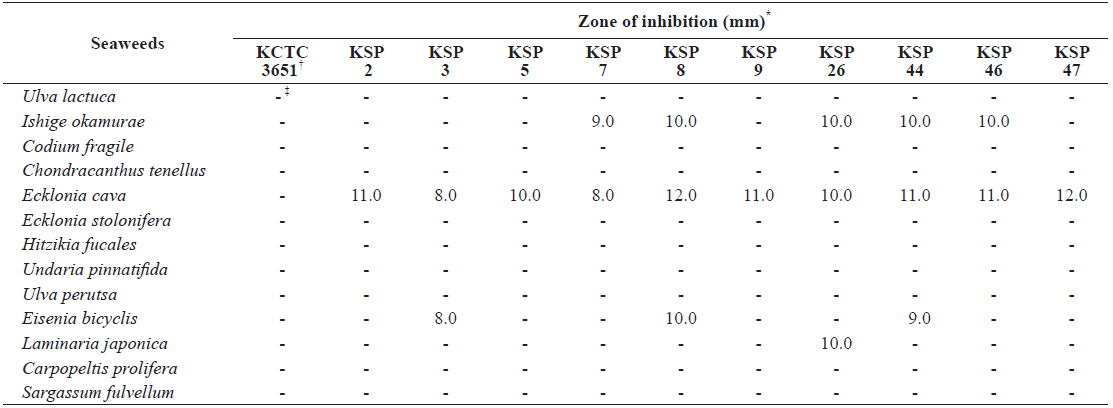
To evaluate the antibacterial activity of seaweed extracts toward
[Table 1.] Disc diffusion assay of seaweeds extract against Streptococcus parauberis strains
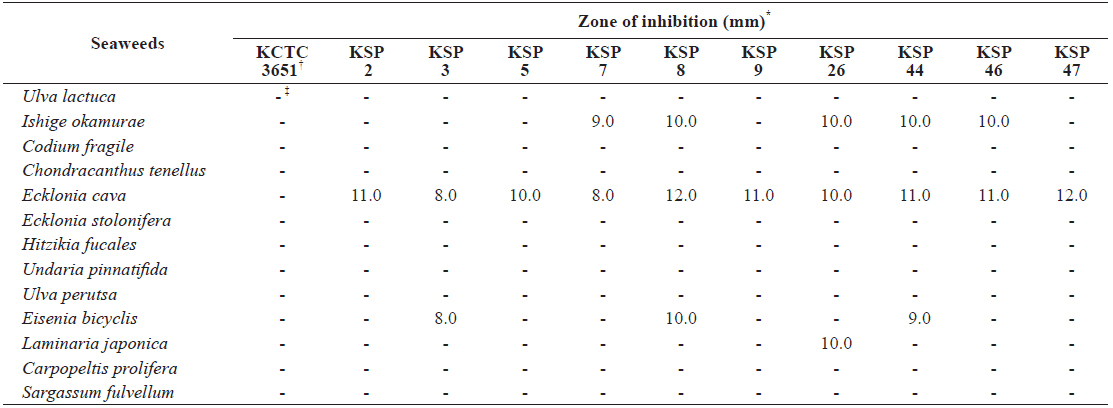
Disc diffusion assay of seaweeds extract against Streptococcus parauberis strains
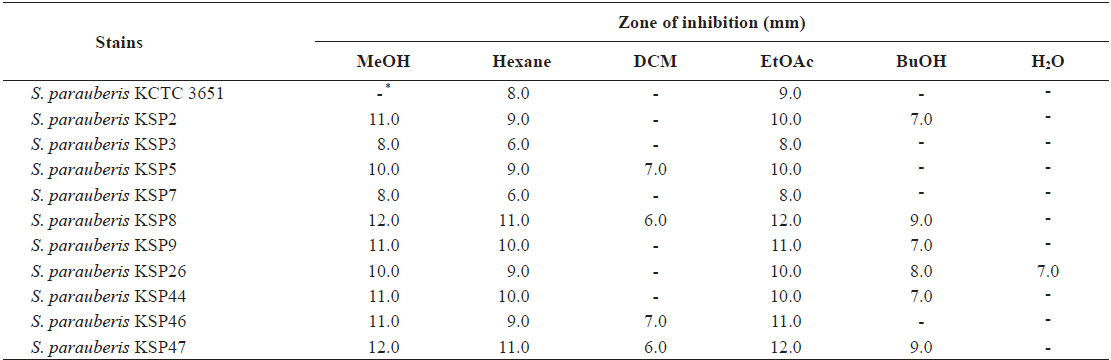
To further investigate the antibacterial effects of
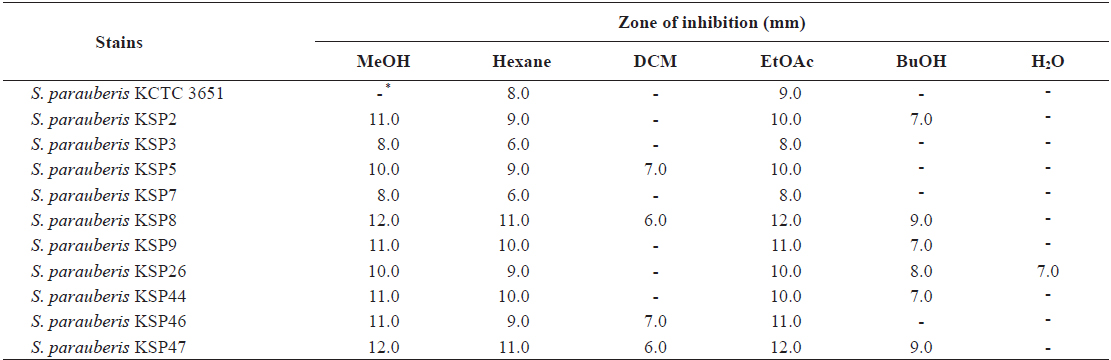
Disc diffusion assay of methanol extract and its solvent-soluble extracts from Ecklonia cava against Streptococcus parauberis strains
From these results, we hypothesized that an antibacterial substance against
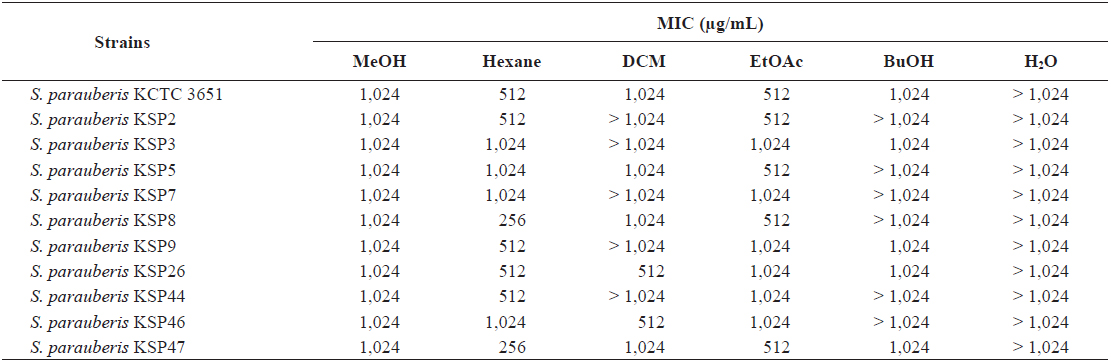
Thus, we performed a quantitative analysis using an MIC assay to precisely evaluate the antibacterial activity of the
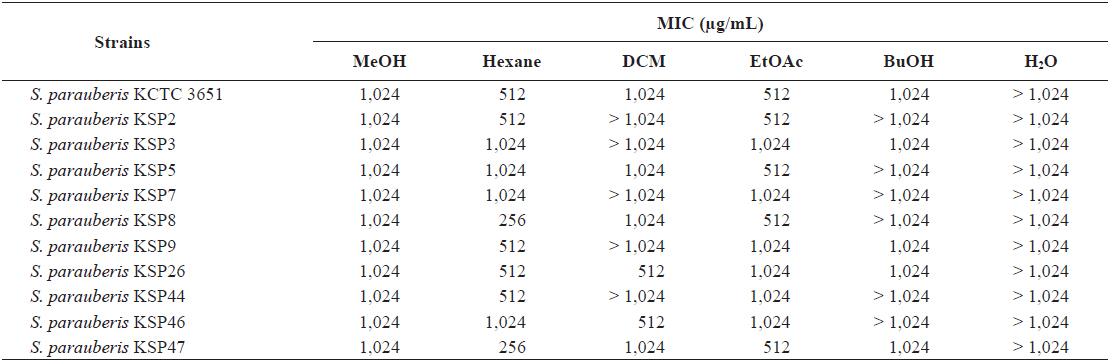
Minimum inhibitory concentration (MIC) of the methanol extract and its soluble fractions from Ecklonia cava against Streptococcus parauberis strains
>
Antibiotic resistance of Streptococcus parauberis strains
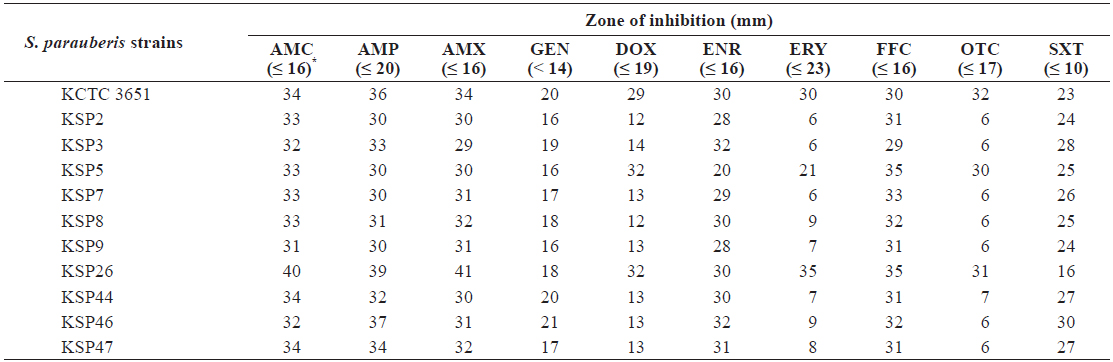
To determine the antibiotic resistance of
[Table 4.] Antibiotic resistance of Streptococcus parauberis strains
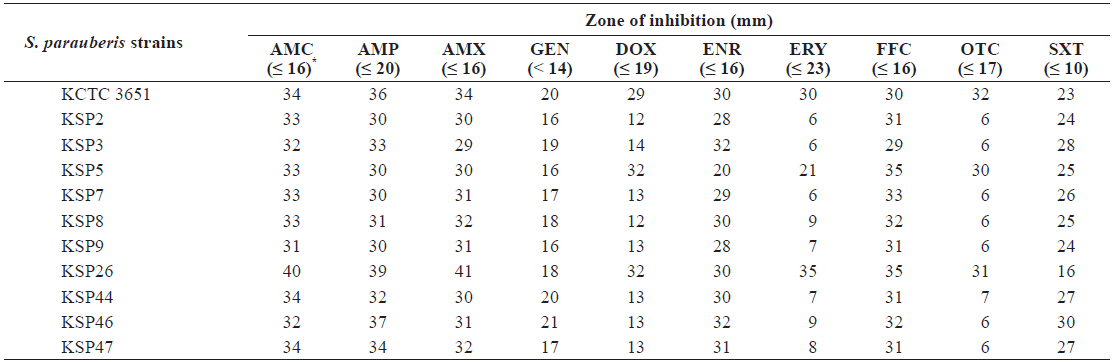
Antibiotic resistance of Streptococcus parauberis strains
The results obtained in this study are consistent with those of previous reports indicating that ERY and OTC have limited efficacy toward
ERY and OTC were used in an MIC assay with the antibiotic-resistant
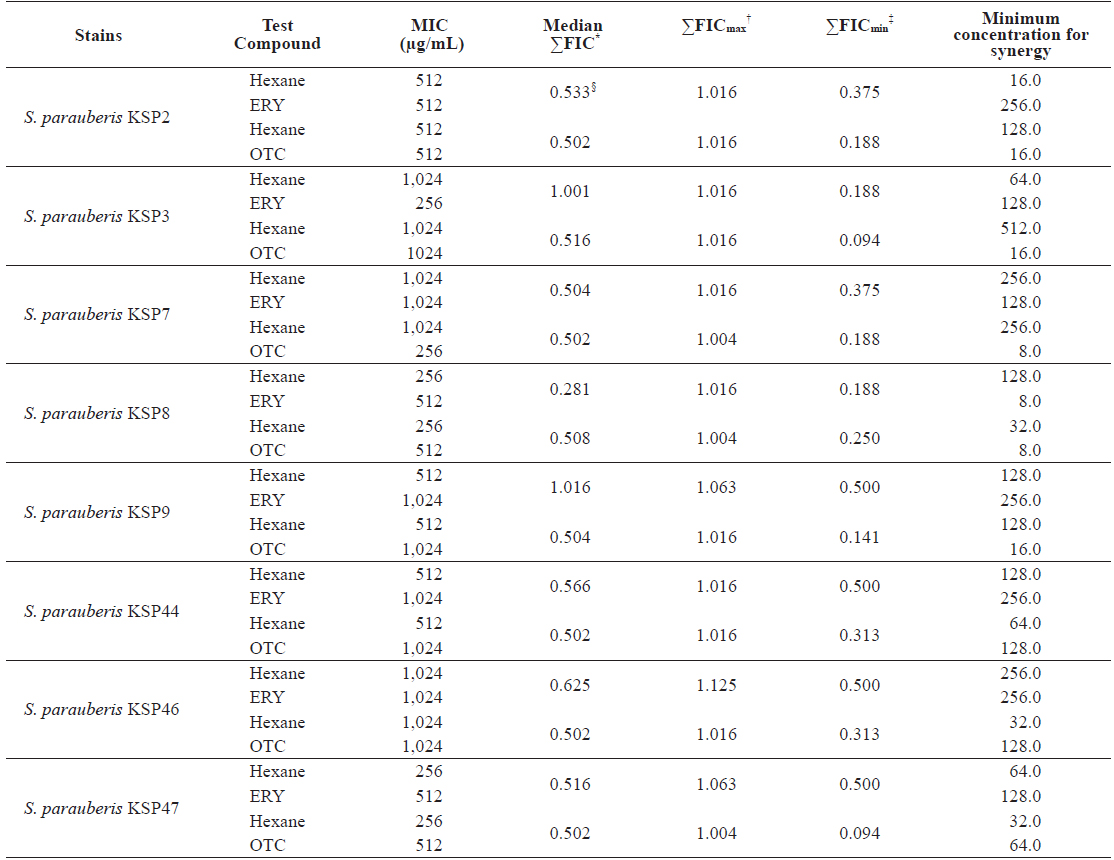
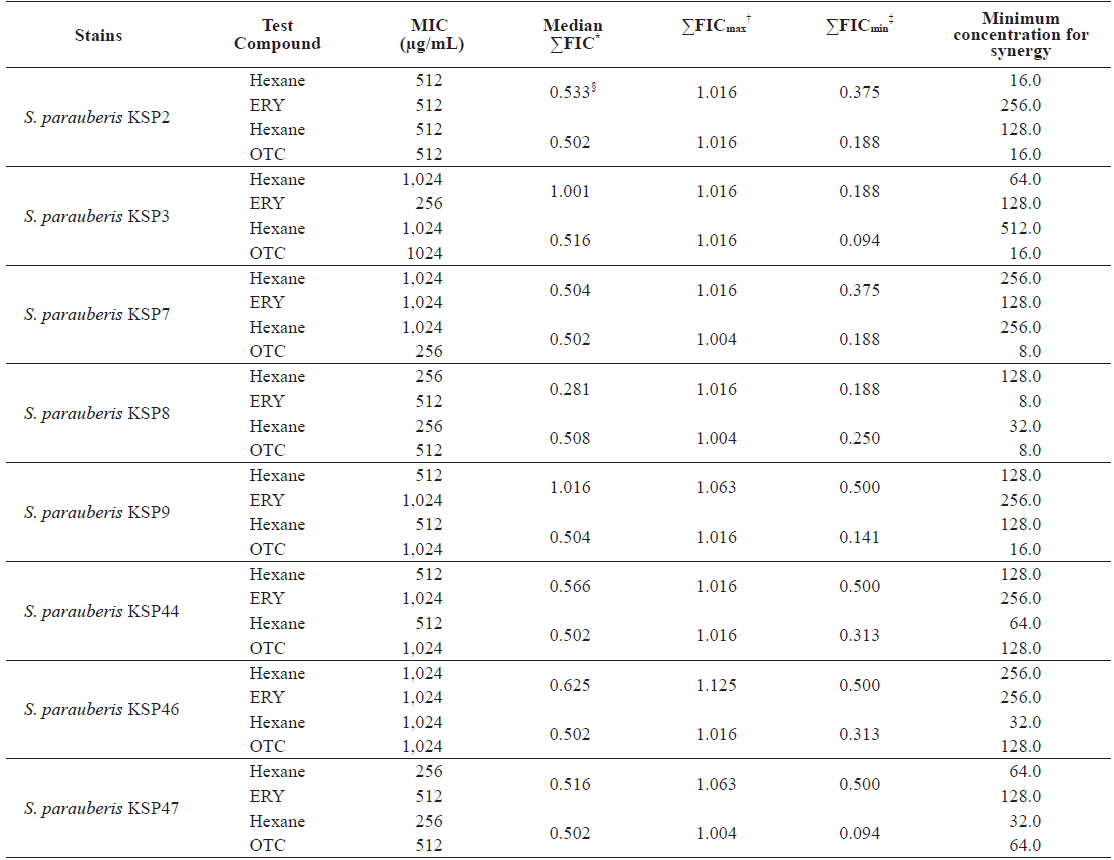
Minimum inhibitory concentrations (MIC) and fractional inhibitory concentration (FIC) indices of the n-hexane (Hexane) fraction of Ecklonia cava in combination with erythromycin (ERY) and oxytetracycline (OTC) against Streptococcus parauberis
>
Synergistic effects of the E. cava Hexane fraction and antibiotics against S. parauberis
One effective strategy for overcoming antibiotic resistance is to restore the activity of the antibiotic. This has been demonstrated by the use of antibiotics in combination with antibacterial agents derived from natural resources (Eom et al., 2014; Lee et al., 2014; Nshimiyumukiza et al., 2015). Here, we evaluated the interaction between the Hexane fraction of an
As shown in Table 5, the MIC values for ERY and OTC in combination with the Hexane fraction were markedly reduced, with 64-fold inhibition against the antibiotic-resistant
FIC indices of ERY and OCT in combination with the Hexane fraction are presented in Table 5. For all strains, the Hexane fraction and ERY combination resulted in a ΣFICmin range of 0.118 to 0.500 and a ΣFICmax range of 1.016 to 1.125. The Hexane fraction and OTC combination resulted in a ΣFICmin range of 0.094 to 0.313 and a ΣFICmax range of 1.004 to 1.016 for all strains. These results suggest that the Hexane fraction had greater synergistic effects in combination with OTC than with ERY. In addition, the median ΣFIC of the OTC and Hexane fraction combination ranged from 0.502 to 0.516. As reported by Lee et al. (2014), the synergistic ranges of median ΣFIC < 1 were observed for all combinations of OTC and the Hexane fraction against the antibiotic-resistant
Despite the multitude of studies on the synergistic effects of natural compounds in combination with antibiotics to treat human pathogenic bacteria, few studies have focused on using this therapy to treat fish pathogens. Although the use of antibiotics is an effective way to control fish pathogenic bacteria in aquaculture farms, it may result in increased antibiotic resistance (Eom et al., 2013). Therefore, restoring the antibacterial activity of conventional antibiotics by combining them with natural compounds is an attractive approach. Natural products can increase the antibacterial efficacy of antibiotics without increasing antibiotic resistance among the bacterial pathogens in aquatic animals. In this study, we demonstrated that an