Tyrosinase inhibitors are an important component of cosmetic products. Our previous studies have proposed that eckol isolated from the brown alga Ecklonia cava, can be explored as a tyrosinase inhibitor. However, cellular activities and mechanism of action of eckol remain unknown. Therefore, the current study analyzed the eckol binding modes using the crystal structure of Bacillus megaterium tyrosinase. The effects of eckol on melanin synthesis induced by α-melanocyte stimulating hormone in B16F10 melanoma cells were also investigated. We predicted the 3D structure of tyrosinase and used a docking algorithm to simulate binding between tyrosinase and eckol. These molecular modeling studies were successful (calculated binding energy value, -115.84 kcal mol-1) and indicated that eckol interacts with Asn205, His208, and Arg209. Furthermore, eckol markedly inhibited tyrosinase activity and melanin synthesis in B16F10 melanoma cells. We also found that eckol decreased the expression of tyrosinase, tyrosinase-related protein (TRP) 1, and TRP2. These results indicate that eckol is a potent inhibitor of melanogenesis, and this finding may be useful for the development of novel pharmaceutical and cosmetic agents.
Tyrosinase (monophenol monooxygenase) is a copper containing, mixed-function polyphenol oxidase (Mallavadhani et al. 1998). Widely distributed in microorganisms, animals, and plants, tyrosinase plays a central role in melanin biosynthesis by catalyzing the hydroxylation of tyrosine to form 3,4-dihydroxyphenylalanine (L-DOPA, monophenolase activity), and L-DOPA to DOPA quinone (diphenolase activity) (Cooksey et al. 1997).
Excessive melanin biosynthesis due to tyrosinase overexpression leads to skin disorders, such as age spots, freckles, melisma, and malignant melanoma (Prezioso et al. 1992, Chen and Kubo 2002). Thus, tyrosinase inhibition is a major strategy for the treatment hyperpigmentation. Further, many tyrosinase inhibitors have been used in cosmetic and pharmaceutical products to prevent melanin overproduction in the epidermis. However, only a few inhibitors, including kojic acid and arbutin, are used in therapeutic and cosmetic agents, because of various safety concerns and low whitening bioactivity (Maeda and Fukuda 1996, Battaini et al. 2000, Solano et al. 2006, Parvez et al. 2007). Therefore, it is necessary to search for novel and effective tyrosinase inhibition. Recently, several groups have shown that metabolites biosynthesized by plants are a promising source of novel compounds (Sirat et al. 2010).
Our previous studies have suggested that eckol, an
The brown alga
Eckol was isolated from
The purity of eckol was >95%, based on the peak area of all components absorbed at each specific wavelength in the HPLC analysis. Eckol was dissolved in dimethyl sulf-oxide (DMSO) and used for experiments after adjusting the final concentration of DMSO in the culture medium to <0.01%.
>
Kinetic analysis of tyrosinase inhibition
Various concentrations of L-tyrosine (0.5 to 1.5 mM), 20 μL of aqueous mushroom tyrosinase solution (1,500 units), and 50 mM potassium phosphate buffer (pH 6.5) were added to a 96-well plate in a total volume of 200 μL, in the presence or absence of eckol. Using a microplate reader, the initial rate of dopachrome formation was determined by measuring the increase in absorbance at 492 nm. The Michaelis constant (
>
Molecular docking of tyrosinase
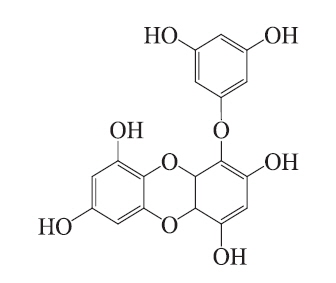
Molecular docking is an application wherein molecular modeling techniques are used to predict how a protein (enzyme) interacts with small molecules (ligands) (Fradera et al. 2000, Gopalakrishnan et al. 2005, Perola 2006). The ability of a protein to interact with small molecules plays a major role in the dynamics of that protein, which may enhance or inhibit its biological function. In the present study, we evaluated eckol docking in the active site of mushroom tyrosinase. The crystal structure of tyrosinase (PDB: 3NM8) was obtained from the Protein Data Bank (PDB, http://www.pdb.org). We performed the docking studies using CDOCKER in Accelrys Discovery Studio 3.0 (Accelrys, Inc., San Diego, CA, USA). The ligand structure of the candidate tyrosinase inhibitor is described in Fig. 1. To prepare for the docking procedure, we performed the following steps: 1) conversion of the 2D structure into a 3D structure; 2) calculation of charges; and 3) addition of hydrogen atoms using the flexible docking program.
B16F10 cells obtained from the Korea Cell Line Bank were grown in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, penicillin (100 U mL-1) and streptomycin (100 μg mL-1). Cells were maintained at 37°C in a 5% CO2 incubator. B16F10 cells were cultured in 24-well plates for melanin quantification and enzyme activity assays.
Cell viability was quantified through a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which measures mitochondrial activity in viable cells. B16F10 cells were seeded (1 × 105 cells mL-1) with various concentrations of eckol (25, 50, and 100 μM) and incubated for up to 72 h prior to MTT treatment. An MTT stock solution (50 μL; 2 mg mL-1 in phosphate buffered saline [PBS]) was added to each well to achieve a total reaction volume of 250 μL. After 4 h of incubation, the plates were centrifuged for 10 min at 2,000 rpm, and the supernatants were aspirated. The formazan crystals in each well were dissolved in DMSO. The amount of purple formazan was assessed by measuring the absorbance at 540 nm.
>
Measurement of cellular tyrosinase activity
Tyrosinase activity was estimated by measuring the rate of L-DOPA oxidation (Kim et al. 2002). Cells were seeded in 24-well dishes at a density of 1 × 105 cells mL-1. B16F10 cells were incubated in the presence or absence of 0.1 μM α-melanocyte stimulating hormone (α-MSH), and were then treated for 72 h with various concentrations of eckol. The cells were washed, lysed in 100 μL of 50 mM sodium phosphate buffer (pH 6.5) containing 1% Triton X-100 and 0.1 mM phenylmethylsulfonyl fluoride, and then frozen at -80°C for 30 min. After being thawed and mixed, the cellular extracts were placed in a 96-well plate, and the absorbance at 492 nm was recorded every 10 min for 1 h at 37°C using an enzyme linked immunosorbent assay plate reader.
>
Measurement of cellular melanin contents
Cellular melanin content was determined as previously described, with modifications (Bilodeau et al. 2001). The amount of melanin was used as an index for melanogenesis. B16F10 cells (1 × 105 cells mL-1) were seeded in 24-well dishes and incubated in the presence or absence of 0.1 μM α-MSH. The cells were then incubated for 72 h with various concentrations of eckol. The samples were washed with PBS and dissolved in 100 μL of 1 N NaOH. The samples were incubated at 60°C for 1 h and mixed to solubilize the melanin. The amount of melanin was assessed by measuring the absorbance at 405 nm.
B16F10 cells were treated with the indicated concentrations of eckol and harvested. The cell lysates were prepared with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, and 1 mM ethylenediaminetetraacetic acid). Cell lysates were cleared via centrifugation, and the protein concentration was determined using a bicinchoninic acid assay protein assay kit. Proteins (30 μg) were subjected to electrophoresis on 10 or 12% sodium dodecyl sulfate–polyacrylamide gels, and were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated with primary antibodies against tyrosinase, tyrosinase-related protein (TRP) 1, TRP2, and β-actin in TTBS (25 mM Tris-HCl, 137 mM NaCl, 0.1% Tween 20, pH 7.4) containing 0.5% nonfat dry milk for 1 h. The membranes were then washed with TTBS and incubated with secondary antibodies. Signals were developed using an enhanced chemiluminescence Western blotting detection kit and exposed to X-ray films.
All data are presented as the mean ± standard deviation. Significant differences between the groups were determined using the unpaired Student’s t-test. A p-value less than 0.05 was considered statistically significant.
>
Inhibitory type and constant of eckol
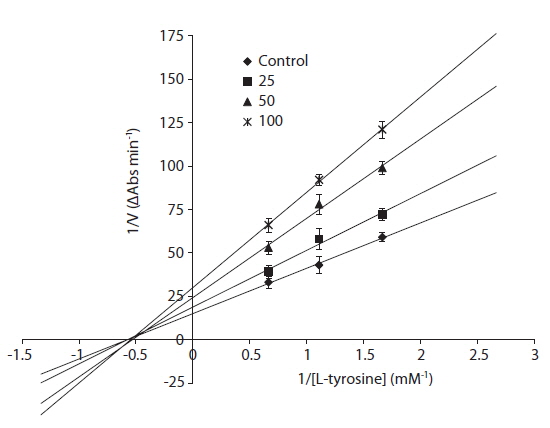
The inhibitory mechanism of eckol was determined using a Lineweaver-Burke double reciprocal plot (Fig. 2). The data, displayed as a plot of 1/V versus 1/[S], gave four straight lines with different slopes and a horizontal line that intersected at the same point. Eckol functioned as a non-competitive inhibitor, which means that eckol can bind tyrosinase to generate an inhibited complex, regardless of whether or not a substrate molecule is bound.
>
Molecular docking of tyrosinase
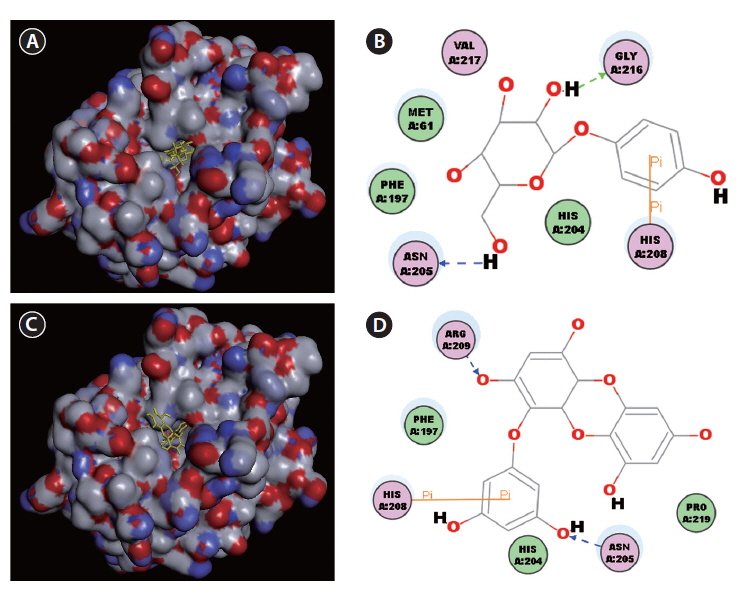
Docking studies were performed to gain insight into the probable binding conformation of eckol, and to compare eckol binding with binding of the commercial tyrosinase inhibitor, arbutin (Fig. 3). Docking of the tyrosinaseligand complexes was performed with eckol or arbutin stably bound to tyrosinase in Discovery Studio (DS) 3.0 (Fig. 3A-D). For arbutin, the predicted binding site was formed by the following resides: Asn205, His208, and Gly216 (Fig. 3A & B). In the case of eckol, the binding site Asn205, His208, and Arg209 (Fig. 3C & D). These molecular docking studies suggest that Asn205 and His208 are critical residues responsible for tyrosinase inhibition. Moreover, the binding energy values of eckol and arbutin obtained from the DS 3.0 binding energy program were -115.84 and -111.69 kcal mol-1, respectively. These results indicated that the tyrosinase binding energy of eckol was more stable and stronger than that of the commercial inhibitor, arbutin. predicted by DS 3.0 was formed by the following resides:
>
Effects of eckol on tyrosinase activity and melanin synthesis in α-MSH-stimulated B16F10 cells
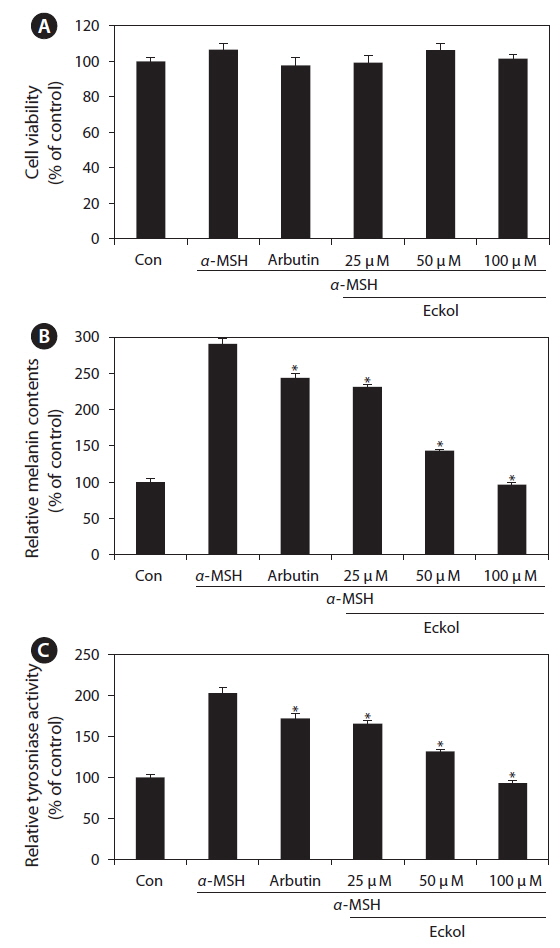
We first examined the cytotoxic effect of eckol on B16F10 cells treated with various concentrations of eckol (25-100 μM). As shown in Fig. 4A, eckol was not cytotoxic at the concentrations tested. In addition, arbutin and α-MSH also exhibited no cytotoxic effects in B16F10 cells. To verify the inhibitor effect of eckol on α-MSHmediated melanogenesis, we determined the quantity of intracellular melanin and tyrosinase activity in the presence of α-MSH. As shown in Fig 4B, eckol substantially decreased α-MSH-induced cellular melanin content in a dose-dependent manner, as compared to cells treated with α-MSH alone. Likewise, eckol also reduced α-MSH-induced intracellular tyrosinase activity in a dose-dependent manner (Fig. 4C). Moreover, 350 μM arbutin had a weaker effect on tyrosinase activity than 50-100 μM eckol. These results suggest that eckol downregulated tyrosinase activity, and that this inhibitory effect may lead to decreased cellular melanin synthesis in B16F10 cells.
>
Effect of eckol on tyrosinase-related protein expression inα-MSH-stimulated B16F10 cells
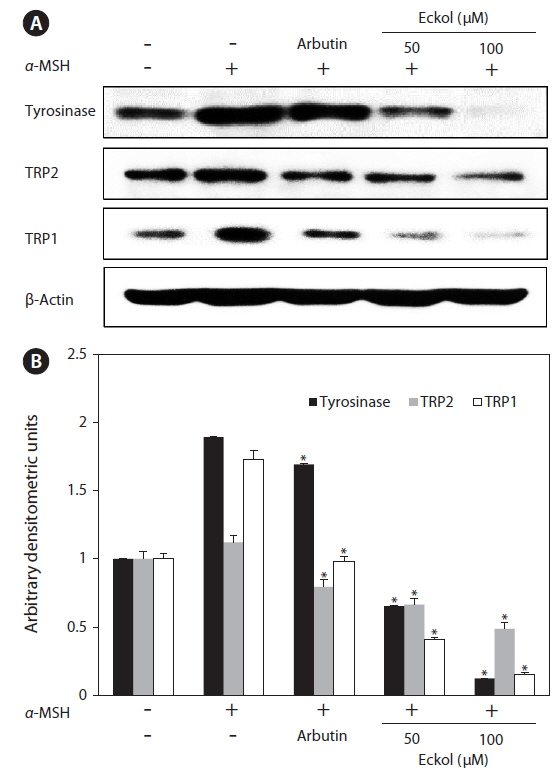
In order to explore the mechanisms underlying eckolmediated inhibition of melanin synthesis, we determined the effects of eckol on the expression level of signaling molecules involved in melanin synthesis. B16F10 cells were exposed to eckol in the presence of α-MSH, and changes in the expression of tyrosinase-related proteins were analyzed by western blot analysis. As shown in Fig. 5, eckol effectively reduced α-MSH-induced expression of tyrosinase, TRP1, and TRP2, and it had a greater effect than arbutin on protein expression. These results suggest that eckol inhibited tyrosinase activity and melanin synthesis through downregulation of tyrosinase-related protein expression.
Melanin plays an important role in preventing ultraviolet light-induced skin damage (Nofsinger et al. 2002). However, overproduction or dysregulation of melanin can lead to skin hyperpigmentation, wrinkling, melisma, and skin cancer (Briganti et al. 2003). Tyrosinase has long been known to be essential for melanization. In vertebrates, tyrosinase, the enzyme responsible for the initial steps of melanin synthesis, is closely associated with specialized organelles called melanosomes, which are found in melanocytes (Chakraborty and Chakraborty 1993). Many commercial tyrosinase inhibitors are used in cosmetics and medical applications to treat skin and pigment abnormalities. Unfortunately, the continuous use of such commercial agents should be limited because they may induce cytotoxicity and adverse side effects. For the these reasons, there has been increased interest in identifying highly effective natural compounds with non-deleterious side effects to replace currently used commercial tyrosinase inhibitors. Many plant extracts and compounds purified from natural products have been investigated for their effects on tyrosinase and melanogenesis (Arung et al. 2011, Chan et al. 2011, Park et al. 2011). In the present study, we found that eckol is a highly effective inhibitor of melanin synthesis. Further, we showed that eckol is more potent than the commercial agent, arbutin.
A kinetic study evaluating the inhibition of mushroom tyrosinase by eckol showed that this compound behaves as a non-competitive inhibitor, as shown by a Lineweaver-Burk kinetic analysis. To understand the mechanism underlying the interaction between tyrosinase and eckol, and to explore their binding, a docking study was performed using the CDOCKER function available in DS 3.0. These docking studies yielded critical information concerning the operation of the inhibitor that interacted with binding pocket of tyrosinase. Ligand-enzyme interaction analysis found that eckol interacted with Asn205, His208, and Arg209, which differed from arbutin, which bound Asn205, His208, and Gly216 in the active site. Phenolic compounds have been studied as de-pigmenting agents, because their chemical structures are similar to tyrosine. It has been suggested that the presence of a hydroxyl group and of an electron donator group in the phenol ring is a primary requirement to effectively serve as a tyrosinase substrate (Fenoll et al. 2000). In the present study, eckol was a more effective inhibitor of tyrosinase than arbutin, implying that the number of hydroxyl groups attached to the benzene ring plays an important role in tyrosinase inhibition.
Melanin production correlates directly with the activity and protein levels of tyrosinase (Maeda et al. 1997). Therefore, to determine the effect of eckol on α-MSH-induced melanogenesis, we investigated whether eckol could inhibit melanin synthesis in the presence of α-MSH. We found that eckol inhibited melanin synthesis and tyrosinase activity in a dose-dependent manner, without inducing cytotoxicity. These results suggest that eckol may downregulate tyrosinase activity and inhibit cellular melanin synthesis in B16F10 cells. In general, melanogenesis is regulated by tyrosinase, TRP1, and TRP2 (Kameyama et al. 1995). Thus, we next evaluated the effect of eckol on the expression of tyrosinase, TRP1, and TRP2 to verify the inhibitory effect eckol on melanogenesis. We found that eckol significantly suppressed tyrosinase, TRP1, and TRP2 expression in α-MSH-stimulated B16F10 cells. These findings suggested that eckol inhibits melanogenesis by suppressing tyrosinase, TRP1, and TRP2 expression.
In conclusion, we isolated eckol, a phlorotannin compound found in