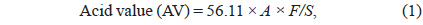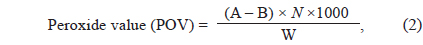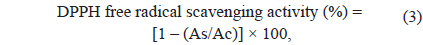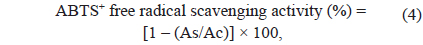Seaweed Sargassum horneri extracts were obtained using supercritical carbon dioxide (SC-CO2) and different solvents. SC-CO2 was kept at a temperature of 45℃ and pressure of 250 bar. The flow rate of CO2 (27 g/min) was constant during the entire 2-h extraction period, and ethanol was used as a cosolvent. Six different solvents [acetone, hexane, methanol, ethanol, acetone mix methanol (7:3), and hexane mix ethanol (9:1)] were used for extraction and agitated by magnetic stirring (250 rpm) in the dark at 25℃ for 20 h; the ratio of material to solvent was 1:10 (w/v). Antioxidant properties of S. horneri extracted using SC-CO2 with ethanol and different solvents have shown good activity. The highest activity belongs to SC-CO2 with ethanol extracted oil, showing DPPH, ABTS, total phenolic content, and total flavonoid levels of 68.38 ± 1.21%, 83.51 ± 1.25%, 0.64 ± 0.02 mg/g, and 5.57 ± 0.05 mg/g, respectively. The S. horneri extracts showed a significant correlation between the antioxidant activity and phenolic content. Based on these results, the SC-CO2 extract (ethanol) of the seaweed extract from brown seaweed may be a valuable antioxidant source.
Marine algae are an important resource of bioactive compounds since they produce a great variety of secondary metabolites characterized by a broad spectrum of biological behavior such as antibacterial, antioxidant, anticancer, anticoagulant, and antiviral properties (Vairappan et al., 2001; Athukorala et al., 2007). Seaweeds are of nutritional interest since they are a low calorie food rich in vitamins, minerals, proteins, polyphenols, polysaccharides, and dietary fibers (Burtin, 2003; MacArtain et al., 2007). Several
The use of supercritical CO2 (SC-CO2) offers numerous potential advantages over conventional extraction processes such as a reduced extraction time and organic solvent volume, and more selective extractions. Supercritical fluids have been gaining increasing attention as environmentally friendly solvents and attractive reaction media for a variety of applications. They are cheap, nontoxic, nonflammable, nonexplosive, and offer essential advantages compared to other substances, particularly in the field of “green chemistry” (Lang et al., 2001; Rogalinski et al., 2008).
CO2 is an attractive supercritical solvent with a moderate critical temperature (31.1℃) and pressure (73.8 bar), and it is nonflammable, nontoxic, and inert. In recent years, the use of SC-CO2 for removing organic compounds from different liquid and solid matrices has attracted a great deal of attention. This technique has some advantages over conventional separation techniques, largely due to the unique physical properties of SC-CO2. SC-CO2 offers a promising approach for the extraction and fractionation of edible oils containing labile polyunsaturated fatty acids (PUFAs) and lipid-soluble bioactive compounds (Jose et al., 2008).
In this study, we extracted oil from
The brown seaweed
After washing fresh
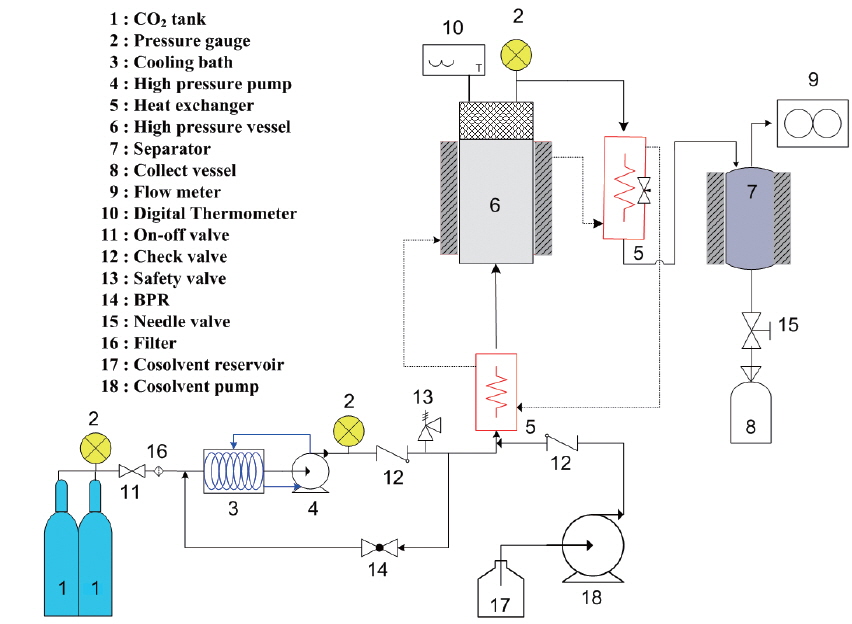
The setup of a laboratory-scale SC-CO2 extraction process is shown in Fig. 1.
Extractions were performed using six different solvents [acetone, hexane, methanol, ethanol, acetone mix methanol (7:3), and hexane mix ethanol (9:1)]. A total of 20 g of freeze dried raw S. horneri with 100 mL of solvent was placed into the beaker and stirred for 20 h using a magnetic stirrer at 25℃ and 300 rpm. After extraction, the hexane solution was filtered using filter paper and evaporated in a rotary vacuum evaporator (Eyela N-1100; Tokyo Rikakikai Co., Ltd.) at 40℃. The remaining residue was dried using a dry oven at 40℃ for 6 h, after which the oil obtained was stored at 4℃ until further use.
The AV was assessed according to the method described by Ping et al. (2008). A total of 1 g of sample was dissolved in 100 mL of ether:ethanol (1:1, v/v) by shaking, after which phenolphthalein (as an indicator) was added drop wise. The AV of oil was analyzed by titration with a 0.1 N KOH–ethanol solution until the pink color persisted for at least 30 s; the AV was calculated using the following equation:
where
The POV was determined according to AOCS method Cd 8-53 (American Oil Chemists’ Society, 1998) using a modified amount of sample. A total of 1 g of seaweed oil was dissolved in 6 mL of a 3:2 acetic acid: chloroform solution. Then, 0.1 mL of a saturated potassium iodine (KI) solution was added to the mixture and allowed to stand with occasional shaking for 1 min. Distilled water (6 mL) was immediately added to the solution and allowed to stand. The solution was titrated with 0.1 N of sodium thiosulfate until the yellow iodine color almost disappeared. Next, 0.4 mL of a starch indicator solution was added by shaking to extract iodine from the chloroform layer, and again titrated until the blue color disappeared. A blank determination was performed with the same procedure. POVs were expressed as mill equivalents of a peroxide/1,000 g sample:
where
>
DPPH free radical scavenging assay
The DPPH radical scavenging capacity of seaweed extract was determined based on the method described by Cai et al. (2006) with minor modifications. A total of 3.9 mL of ethanolic DPPH radical (60 μM) was first mixed with 0.1 mL of seaweed extract (1 g/10 mL) or ethanol (as a control) and stored in a dark environment at room temperature for 30 min. Subsequently, the absorbance of the seaweed extract and control was measured against ethanol (as the blank) at 517 nm using a UV-spectrophotometer (UVmini-1240; Shimadzu Co., Japan). The absorbance measurements of the seaweed extract and control were performed in triplicate. The percentage of DPPH free radical scavenging capacity was calculated using the following formula:
where As is the absorbance of the seaweed extract at 517 nm and Ac is the absorbance of the control at 517 nm. Blank and samples (1.00 mg/mL standard trolox) were analysed as described above.
>
ABTS+ radical scavenging capacity assay
An ABTS+ radical scavenging capacity assay was performed according to procedures described by Cai et al. (2006). ABTS+ radical solution was first prepared by mixing 10 mL of a 7 mM ABTS+ radical solution with 10 mL of a 2.45 mM potassium persulfate solution in an amber bottle. Subsequently, the ABTS+ radical solution was allowed to stand in a dark environment at room temperature for 12–16 h to yield a dark blue solution. The ABTS+ radical solution was diluted with denatured ethanol until its absorbance was equilibrated to 0.70 ± 0.02 at 734 nm before use. A total of 3.9 mL of ABTS+ radical solution was first mixed with 0.1 mL of seaweed extract (1 g/10 mL) or ethanol (as control) and stored in a dark environment at room temperature for 6 min. Subsequently, the absorbance of the seaweed extract and control was measured against ethanol (as the blank) at 734 nm using a UV-spectrophotometer (UVmini-1240; Shimadzu Co., Japan). The absorbance measurements of the seaweed extract and control were performed in triplicate. The percentage of ABTS+ free radical scavenging activity was calculated using the following formula:
where As is absorbance of the seaweed extract at 734 nm and Ac is absorbance of the control at 734 nm. The Blank and samples (1.00 mg/mL standard trolox) were analyzed as described above.
>
Total phenolic content (TPC) assay
The TPC of seaweed extract was determined using the Folin–Ciocalteu colorimetric method according to Li et al. (2008) with minor modifications. A total of 1 mL of (1 g/10 mL) the seaweed extract was mixed with 1 mL of 1:10 (v/v, in deionized water) diluted Folin–Ciocalteu reagent (FCR). After 4 min, 800 μL of the sodium carbonate solution (7.5%, w/v) was added to the mixture. The mixture was then vortexed for 5 s and stored at room temperature in a dark environment for 2 h. The blank was also prepared by replacing 1 mL of deionized water. The absorbance of the mixture was measured at 765 nm against the blank using a UV-spectrophotometer (UVmini 1240; Shimadzu Co.). The measurements were performed in triplicate. Gallic acid was used for calibration of the standard curve (
>
Total flavonoid content (TFC) assay
The TFC of the seaweed extract was estimated using procedures described by Ozsoy et al. (2007). A total of 1.25 mL of deionized water was added to 0.25 mL of seaweed extract (1 g/10 mL), followed by the addition of 75 mL of a 5% (w/v) sodium nitrite solution. The mixture was allowed to stand for 6 min and 150 μL of a 10% (w/v) aluminium chloride solution was added. The mixture was allowed to stand for another 5 min, and 0.5 mL of a 1 M sodium hydroxide solution and 275 μL of deionized water were added accordingly. Subsequently, the mixture was vortexed for 5 s and its absorbance was determined at 510 nm against a blank using a UV-spectrophotometer (UVmini-1240; Shimadzu Co.). Measurements were performed in triplicate. The blank was prepared by replacing 0.25 mL of the undiluted seaweed extract with 0.25 mL of deionized water. Catechin was used for calibration of the standard curve (
All experiments were performed in triplicate. Experimental values were expressed as the mean ± standard deviation (SD). Differences were considered significant using Tukey’s test and
>
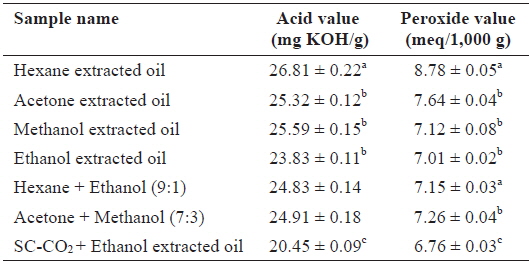
Comparison of the AV and POV
The quality of oil deteriorates at different rates depending on the production and storage conditions (Kamal-Eldin and Yanishlieva, 2002). The AV and POV contents of oil extracted using SC-CO2 with ethanol and other solvents are shown in Table 1. The AV and POV contents were higher in hexane-extracted oil than in other solvents and SC-CO2 with ethanol-extracted oil. Since the hexane extraction system is open, samples are exposed to increasing amounts of oxygen during the extraction, which explains the increased oxidation. In contrast, exposure to low levels of oxygen during SC-CO2 extracradition caused minimal oxidation. The AV was calculated to determine the acidity of oil, and a low AV is indicative of a high oxidative stability (Essien et al., 2012). In contrast, the POV of an oil or fat is a measurement of rancidity due to autoxidation. The AV and POV of
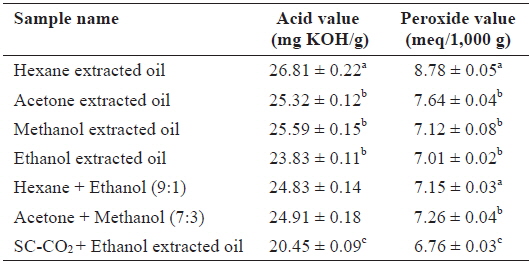
Acid value and peroxide value content of Sargassum horneri oil obtained by SC-CO2 (45℃/250 bar) and different solvent extraction
>
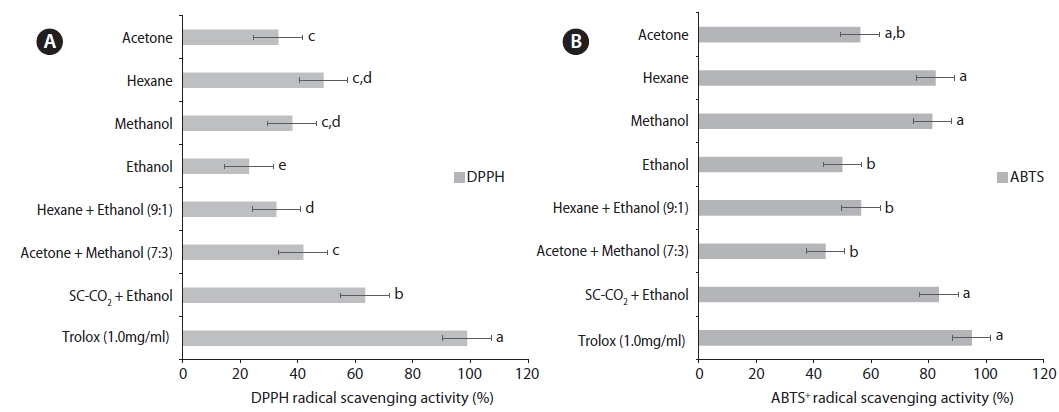
Antioxidant properties of S. horneri DPPH and ABTS+ radical scavenging activity
Due to the presence of different antioxidant components in the crude extracts of biological samples, measuring each antioxidant component separately is relatively difficult. Therefore, several assay methods have been developed and applied to screen and evaluate the total antioxidant activity of such samples. These methods target different mechanisms of the oxidant defense system, i.e., scavenging active oxygen species and hydroxyl radicals, reduction of lipid peroxyl radicals, inhibition of lipid peroxidation, or chelation of metal ions (Pandithurai et al., 2014).
In the present work, the DPPH and ABTS+ radical assay systems were successfully used to evaluate antioxidant activities of the SC-CO2 extraction and various solvent extractions from
The SC-CO2 with ethanol and hexane extracted oil showed the highest DPPH radical scavenging activities of 68.38 ± 1.21% and 48.90 ± 1.01%, respectively (Fig. 2), which were significantly greater than those of the other solvent extractions. The acetone-extracted oil, hexane mix ethanol (9:1)-extracted oil, and ethanol-extracted oil showed relatively poor radical scavenging activity with values of 33.23 ± 1.46%, 32.54 ± 0.89%, and 23.03 ± 1.21%, respectively, while trolox showed 98.11%. These results were consistent with those reported by Hong et al. (2010) for brown and red seaweed. The DPPH method measures the free radical scavenging activity of antioxidants directly from the seaweed extracts, and the ability of a seaweed extract to scavenge the reactive metabolites will inhibit the formation of primary and secondary oxidation products. Devi et al. (2008) reported that DPPH radical scavenging ability differed significantly between the different varieties (ranging from 5% to 72.5%).
The ABTS+ radical cation decolorization test is another method commonly used to assess antioxidant activity. A reduction in color indicates a reduction of ABTS+ radical (Adedapo et al., 2008). The SC-CO2 with ethanol, methanol, and hexane-extracted oil demonstrated the highest ABTS+ radical scavenging activity at 83.52 ± 1.25%, 82.40 ± 1.32%, and 81.31 ± 1.41%, respectively, while trolox showed 95.00% (Fig. 2). The hexane mix ethanol (9:1)-extracted oil (56.45 ± 1.32%), acetone-extracted oil (56.21 ± 1.11%), and ethanol-extracted oil (49.96 ± 1.36%) displayed low activity. The acetone mix methanol (7:3)-extracted oil at 44.10 ± 1.61% exhibited relatively poor radical scavenging activity, clearly showing that SC-CO2 with ethanol can produce a high antioxidant capacity compared to the normal solvent method.
The assay applied in this study was according to the improved technique for the generation of ABTS+ radicals, which involves the direct production of the blue/green ABTS+ radical chromophore though the reaction between the ABTS+ radical and K2S2O8. The higher ABTS+ radical scavenging ability exhibited by n-hexane may occur due to the presence of carotenes or other pigments with long hydrocarbon chains. Previous studies have reported that hexane, chloroform, and methanol extracts of Porphyra yezoensis showed antioxidant activities due to the presence of β-carotene, chlorophyll analogs (pheophytin) and amino compounds (leucine, phenylalanine, and mycosporine-like amino acid). Other reports have claimed that seaweeds contain antioxidant compounds that include pigments such as fucoxanthin and astaxanthin, polyphenols such as phlorotannins, chlorophyll-related compounds, phospholipids, flavonoids, bromophenols, and polysaccharides (Chakraborty et al., 2013). A strong radical scavenging effect on alkyl radicals was reported for
Phenolic compounds are widely distributed in the plant kingdom and have been reported to have several biological activities, including antioxidant properties. Earlier reports revealed that marine seaweed extracts, especially their polyphenols, possess antioxidant activity, with the major active compounds in different seaweed extracts reported to be phlorotannins and fucoxanthin (Hong et al., 2010).
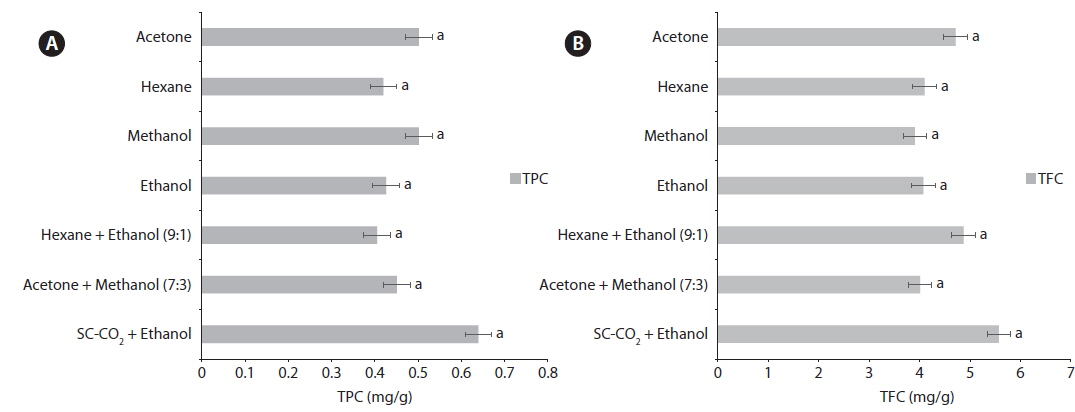
Previous studies have reported that phenolic compounds are the primary contributors to the antioxidant activity of various seaweeds (Zhang et al., 2007). The TPC of the seaweed extracts was calculated using a modified Folin–Ciocalteu method (Fig. 3). The SC-CO2 with ethanol-extracted oil (0.64 ± 0.02 mg/g) displayed a higher TPC than all other seaweed extracted oil. The methanol extracted oil and acetone-extracted oil both showed a high level of phenolics from oil extraction at 0.51 ± 0.02 mg/g and 0.50 ± 0.01 mg/g, respectively. A positive correlation was observed between TPC and the antioxidant activity of different solvent seaweed extracts in previous studies (Zhang et al., 2007).
The TFC of the extracted oils is shown in Fig. 3. The TFCs were grouped into four levels. The first and highest level with the highest value belonged to SC-CO2 with ethanol-extracted oil (5.57 ± 0.05 mg/g). The second level with the middle TFC included the acetone mix methanol (7:3)-extracted oil (4.87 ± 0.02 mg/g) and the acetone-extracted oil (4.71 ± 0.02 mg/g). The final level with the lowest TFC was the methanol-extracted oil (3.91 ± 0.03 mg/g). The effects of solvents on the TFC were similar to that on the TPC. The hexane extracts showed high antioxidant activity, while the TPC and TFC content was not that much higher because some other compounds in the extract may inhibit the high antioxidant activity as an alternative to phenols and flavonoids. The effects of solvents on TFC were similar to that on TPC. This was also described by Do et al. (2014), who indicated that flavonoids are the dominating phenolic group in
This result demonstrated that these
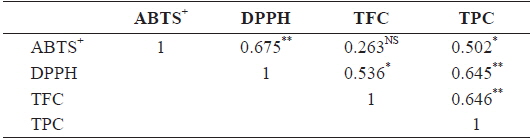
The relationships among antioxidants (DPPH, ABTS+), TPC, and TFC of different solvent extractions are shown in Table 2. Correlations between DPPH and ABTS+ (Pearson’s
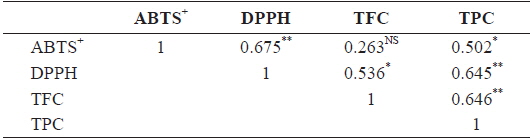
Correlation among DPPH, ABTS+, TFC, TPC based on n=21 means from Sargassum horneri of different solvents extraction
The antioxidant properties of extracted oil were affected by bioactive compounds such as phenolics, flavonoids, and minerals, among others. The growth environment, harvest time, and storage condition also had significant effects on the amount of these compounds (Roh et al., 2008). The majority of antioxidant compounds found in