Bio-imaging and drug carriers for delivery have created a huge demand for crystals. Crystals are fascinating materials that have been grown for a long time but obtaining biocompatible fluorescent crystals is a challenging task. We report on the growth of fluorescent crystals using a carbon dot (C-dot) solution by a hydrothermal process. The crystallization pattern of these C-dots exhibited a unique dendritic structure having a feather-like morphology. The growth temperature and pressure were maintained at 60℃ and 200 mmHg, respectively, for crystal growth. A green fluorescence (under UV light) that was observed in the C-dot solution was retained in the crystals formed from the solution. Cytotoxicity studies on Vero cells revealed the crystals to be extremely biocompatible. These fluorescent crystals are extremely well suited for biomedical and optoelectronic applications.
Since the accidental discovery of carbon dots (C-dots) during the purification of single walled carbon nanotubes [1], they have been studied widely for a vast array of applications [2]. Zero dimensional C-dots are known for their size dependent fluorescence properties [3], bio-compatibility and high water solubility [4], chemical inertness, and low toxicity [5]. These properties of C-dots make them suitable for bio imaging [6], drug delivery [7], photo catalytic activity, and other practical applications [2].
The attractive optical properties of C-dots have motivated researchers to use these tiny carbon nanoparticles to grow fluorescent crystals. Organic nonlinear optical crystals have many applications in biological labelling [8,9]. Fluorescent crystals can be used for bio sensing, optical data storage, document security, detecting forgery [10], and photo induced electron transfer property for light harvesting [11,12]. By exploiting their exceptional properties, C-dots can replace semiconductor metal quantum dots such as CdSe, CdTe, and CdS, which involve extremely harmful precursors [13,14] as well as complex procedures for their synthesis.
Numerous methods of crystal growth such as high temperature growth and low temperature growth [15,16] have been reported for different types of semi-conductor crystals. In the present work, C-dots were synthesized using a microwave assisted method using Sorbitol as a precursor followed by their crystallization. The formation of dendritic crystals from C-dots was observed and explained by a straightforward method using C-dots as compared to the exacting conditions in chemical vapour deposition and electrochemical methods. Fluorescent studies have been performed to assess the optical properties of carbon dendrites. To the best of our knowledge this is the first report on the synthesis of fluorescent crystals grown in a controlled environment using a C-dot solution.
C-dots were synthesized as per our previous report using Sorbitol by microwave assisted heating [17]. The dark brown C-dot solution was then purified by dialysis against nanopure water using a pre-activated dialysis bag (MW cut-off 12-14 kD).
Spectral properties of C-dots were studied by a UV-visible spectrophotometer (Lambda-25, Perkin Elmer, USA) and fluorescence spectroscopy (Perkin Elmer) using a standard quartz cuvette with a path length of 1 cm.
Morphological characteristics of C-dots were studied using transmission electron microscopy (TEM; Carl Ziess, GmbH, Germany). The sample was loaded on a copper grid for the TEM analysis.
The purified C-dots were loaded on clean quartz plates and subjected to vacuum drying under pressure (200 mmHg) for 8 hours at 60℃ (Ganesh Scientific Ltd., India) to initiate crystallization. The morphological studies after crystallization were carried out using an inverted optical fluorescence microscope (Carl Ziess-Axiovert 40).
In order to study the cytotoxicity, C-dot crystals were weighed and re-dissolved in sterile D/W. Cytotoxicity experiments were carried out as per our previous publication using a MTT assay [7].
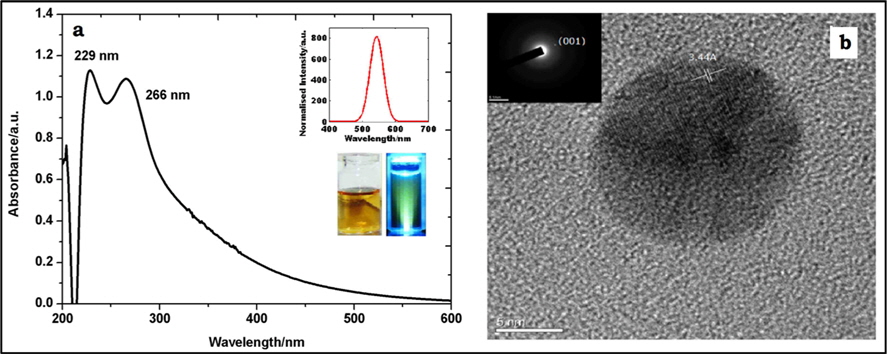
Although the purified (dialyzed) solution was light brown, it exhibited deep green fluorescence under UV light (365 nm), as seen in the inset of Fig. 1a. The origin of fluorescence in C-dots is ascribed to the presence of surface defects and functional groups exposed on the surface (e.g., hydroxyl and carboxylic acid functional groups).
The optical spectra of purified C-dots displayed in Fig. 1a reveal two distinct peaks at 229 and 266 nm, which arise due to π→π* and n→ π* transitions associated with C=C and carbonyl/hydroxyl groups, respectively [18]. Photoluminescence (PL) spectra of C-dots show a sharp peak at 543 nm when excited at 350 nm (inset of Fig. 1a). The appearance of PL may be attributed to the oxygen containing moieties derived from the precursor and functional groups on C-dots [2]. Fig. 1b shows a transmission electron micrographic image of a single C-dot of 7 nm diameter. The C-dots show lattice spacing of 3.44 Å (marked in Fig. 1b). This inter-atomic spacing is equal to the (001) plane spacing of graphite, which confirms the graphitic nature of C-dots and is denoted in the surface area electron diffraction pattern (inset of Fig. 1b).
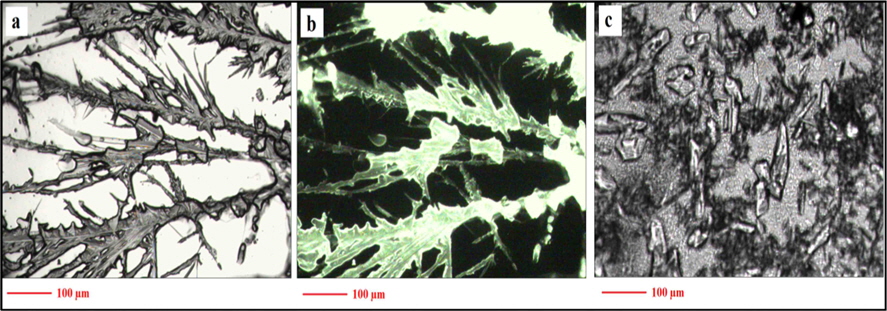
After vacuum drying of the C-dot solution on a quartz plate, a typical feather-like pattern of crystals was observed (Fig. 2a) under an optical microscope. The crystals showed the same green fluorescence when exposed to UV light (Fig. 2b). A dialysed NaOH and ethanol solution, which was used during the fabrication of the C-dots, was also vacuum dried under the same conditions noted in the Materials and Methods section. Fig. 2c shows that the pattern of crystallization is different and small irregularly shaped crystals were observed. Also, no fluorescence was observed under UV light (data not shown). This clearly confirms the deep green fluorescence.
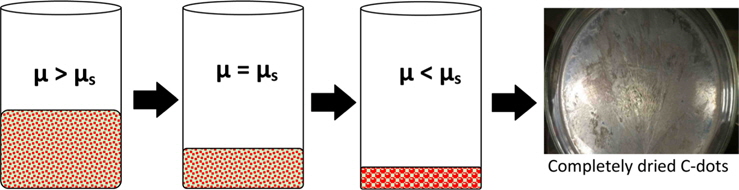
The arrangement of crystals is a stochastic process. The process explained below can be speculated on the basis of classical nucleation theory [19]. The solution contains smaller size C-dots, which have higher free energy with chemical potential µ as compared to a supersaturated solution (chemical potential = µs). This C-dot solution was kept at temperature of 60℃ and pressure of 200 mmHg until it was completely dried. During this process, the density of C-dots in the solution increases as the water evaporates. At the point of critical supersaturation where µ > µs, crystal nucleation is initiated because supersaturated liquids are highly unstable (Fig. 3). As per thermodynamics, the Gibbs free energy of the solution decreases due to evaporation, which initiates the nucleation of C-dots to form crystals [20]. The entropy of the solution decreases, and hence fusion takes place, causing disorder in the surroundings to balance the entropy of the universe [19]. Further research is under way to optimize and realize the effects of parameters such as temperature, pressure, duration etc. on the crystallization.
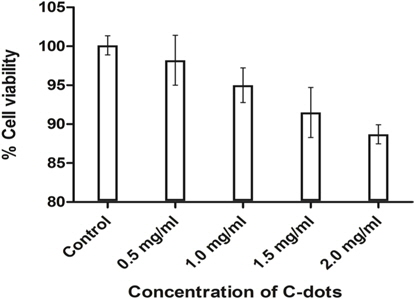
Furthermore, the re-dissolved crystals of C-dots were found to have excellent biocompatibility on Vero cells. The percentage survival of cells was found to be more than 80% at all concentrations used (0.5-2 mg/mL). An initial concentration of 0.5 mg/mL showed negligible cell death whereas 88.7% live cells were observed at the highest concentration used, i.e. 2 mg/mL (Fig. 4). Our results are consistent with previous reports on the non-toxic nature of C-dots [21,22]. These C-dots crystals hence prove to be harmless and can be used in various biological applications.
A simple hydrothermal method was used to grow fluorescent crystals from a C-dot solution at 60℃ and pressure of 200 mmHg. During evaporation of the C-dot solution fascinating structures were formed that showed a consistent feather-like dendritic pattern. Even after the formation of crystals the C-dots retained their fluorescent property. The fluorescence property of the crystals is expected to open up a new area of research in optoelectronics.