In this study, the characterizations of oxide contact hole etching are investigated with C4F8/O2/Ar and CH2F2/C4F8/O2/ Ar plasma. As the percent composition of C4F8 in a C4F8/O2/Ar mixture increases, the amount of polymer deposited on the etched surface also increases because the CxFy polymer layer retards the reaction of oxygen atoms with PR. Adding CH2F2 into the C4F8/O2/Ar plasma increases the etch rate of the oxide and the selectivity of oxide to PR. The profile of contact holes was close to 90°, and no visible residue was seen in the SEM image at a C4F8/(C4F8+O2) ratio of 58%. The changes of chemical composition in the chamber were analyzed using optical emission spectroscopy, and the chemical reaction on the etched surface was investigated using X-ray photoelectron spectroscopy.
SiO2 (oxide) etch processes having high selectivity over photoresist (PR), silicon, and Si3N4 (nitride) are required to manufacture ultra-large-scale integrated-circuit devices. Generally, oxide etching is performed by using fluorocarbon gases to deposit a fluoro-polymer on the underlying silicon or mask materials. This deposit enhances the etching selectivity of oxide over silicon or nitride. However, in the patterning of high-aspect-ratio contact holes or self-aligned contacts, these plasmas have serious problems related to the low selectivity of the underlying or mask material, the micro-loading effect, and the etch stop interrupting the etching [1].
Previous studies focused on either the anisotropic etching or selective etching of oxide over silicon, nitride, or PR using conventional low-density plasma sources. Recently, high-density-plasma (HDP) systems have attracted considerable attention, primarily because HDP sources operate at low pressure and allow independent control of ion flux and ion energy [2-5]. Consequently, the plasma-surface interactions become significant, and surface conditions such as the temperature and cleanliness of the reactor wall play an important role in determining both the gas-phase chemistries and the surface-reaction deposition and etching on the wafer surface.
We studied the fluorocarbon layer formation that appears to dominate the surface reaction in order to understand the etch mechanism for highly selective etching using C4F8 and CH2F2 additive chemistry in contact hole etching. The relationship between gas chemistry and the fluorocarbon film was systematically investigated. Optical emission spectroscopy (OES) and X-ray photoelectron spectroscopy (XPS) were used to quantitatively analyze the gas chemistry and etched surfaces, respectively.
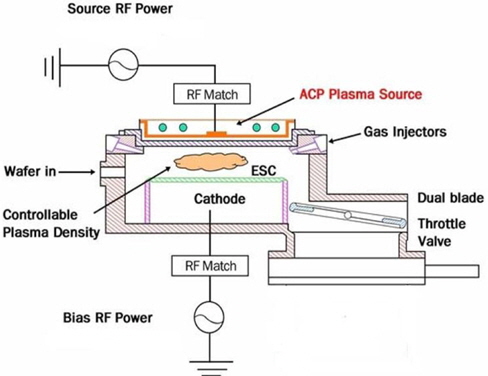
The HDP etching system is an APTC SELEX200, as shown in Fig. 1. Multi-spiral coils and a bushing, connected to the 12.56-MHz RF power generator, are located above a thick horizontal ceramic window. This plasma source shows characteristics of both traditional sources of inductively coupled plasma (ICP) and capacitively coupled plasma (CCP), as well as its own characteristics. Some of the basic properties and process performances were previously reported [6]. A Semisysco smart-EPDTM system of optical emission spectroscopy was used to observe specific plasma, chemical, and wafer behaviors in the process chamber. Through the extraction of the characteristic behaviors from the optical spectrum, a representative value of a certain process can be obtained.
On a 200-mm wafer, 600-nm-thick TEOS (oxide) was deposited using a low-pressure chemical vapor deposition (LPCVD) and PR films were prepared using a spin-coating system. The etch rates of non-pattern oxide and PR were measured as a function of the C4F8/O2 gas mixing ratio and CH2F2 addition into the C4F8/O2/Ar plasma, with the process pressure of 16 Pa, RF power of 100 W, bias power of 1,800 W, and Ar flow rate of 300 sccm. The total flow rate is 331 sccm. The pattern samples were prepared to have contact hole structures by using the KrF photoresist that was used to check the etch profile. The PR thickness for the contact-hole etching mask was 200 nm, and a 58-nm-thick organic bottom anti-reflected coating (OBARC) was used for the photo-mask process.
To analyze the effect of the C4F8/O2 gas mixing ratio and addition of CH2F2 gas into the C4F8/O2/Ar plasma, we investigated the chemical species in the gas phase with an optical emission spectroscopy. The surface analysis of polymer on oxide films was etched in various C4F8/O2 gas mixing ratios and was performed using X-ray photoemission spectroscopy (XPS, VG Scientific ESCALAB 200R). Etched contact hole patterns were characterized using field emission scanning electron microscopy (FE-SEM, FEI Sirion 400) for the etch profile.
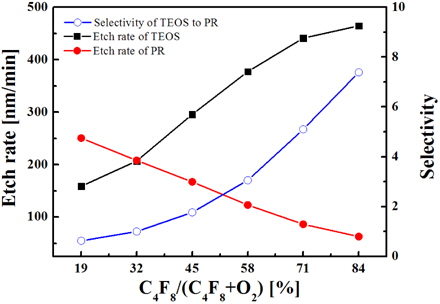
Figure 2 shows the etch rates for both oxide and PR as functions of the C4F8/O2 mixing ratio in the C4F8/O2/Ar plasma. Also, the etch selectivity of oxide over PR is shown. As the C4F8 fractional flow rate increases, the oxide etch rate also increases, while the etch rate of PR decreases. Accordingly, higher etch selectivity is obtained for higher C4F8 concentration in the C4F8/O2/Ar plasma. The behaviors of etch rates can be simply associated with the behaviors of the corresponding active species that include fluorine atoms and CFx radicals for the oxide and oxygen atoms for the PR. However, it is reported that the selective etching of oxide over PR using various fluorocarbon plasmas is influenced not only by the fluxes of active species, but also by the thickness of the fluorocarbon CxFy polymer layer formed over the etched structure. Evidently, an increase in the C4F8/O2 mixing ratio increases the thickness of the fluorocarbon CxFy polymer layer due to both the increasing deposition rate (through the increasing flux of fluorocarbon radicals) and decreasing destruction rate that is supported by the chemical reaction with oxygen atoms. At the same time, the CxFy polymer layer deposited on the oxide can be effectively destroyed by the oxygen released from the oxide. As such, the polymer layer on the PR is expected to be thicker than that for oxide and to play the role of inhibitor for the reaction of oxygen atoms with PR. Together with the decreasing flux of oxygen atoms, the effect of CxFy polymer layer also decreases the etch rate of PR and increases the etch selectivity of oxide over PR with increasing C4F8/O2 mixing ratio.
We used OES analysis to investigate the behaviors of the plasma active species. The emission intensities of F (703.7 nm), CF (247.5 nm), CF2 (251.9 nm), O (844.6 nm), and CO (483.1 nm) radicals were measured as functions of the C4F8/O2 gas mixing ratio [7].
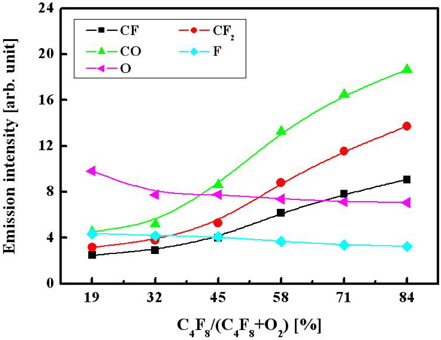
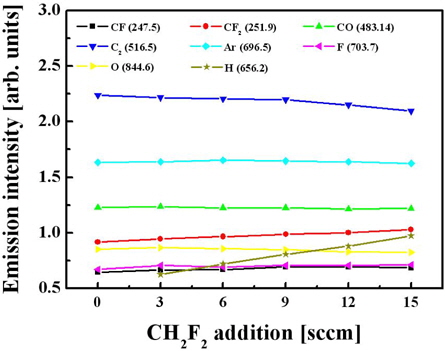
Figure 3 shows that as the C4F8/O2 mixing ratio increases, the emission intensities for both the CF and CF2 radicals increase, but the intensities of the atomic lines corresponding to the O and F atoms decrease. Assuming that the emission intensities are proportional to the volume densities of the corresponding species in a gas phase, the observed changes in plasma composition can result from a combination of several factors. Particularly, increases in both CF and CF2 densities are potentially caused by two effects: (i) the increasing density of C4F8 molecules that are the only source of CFx radicals in our system and (ii) the decrease in oxygen partial pressure, which lowers the destruction rate for CFx radicals (CFx + O → COFx−1 + F) both in bulk plasma and on the etched surface [8]. This also explains the decreasing tendency mentioned for the volume density of F atoms. Finally, comparing the data in Figs. 2 and 3, one can assume that the etching of oxide is controlled more by CFx radicals than by F atoms.
We used XPS measurements to analyze the chemical composition of the etched surfaces.
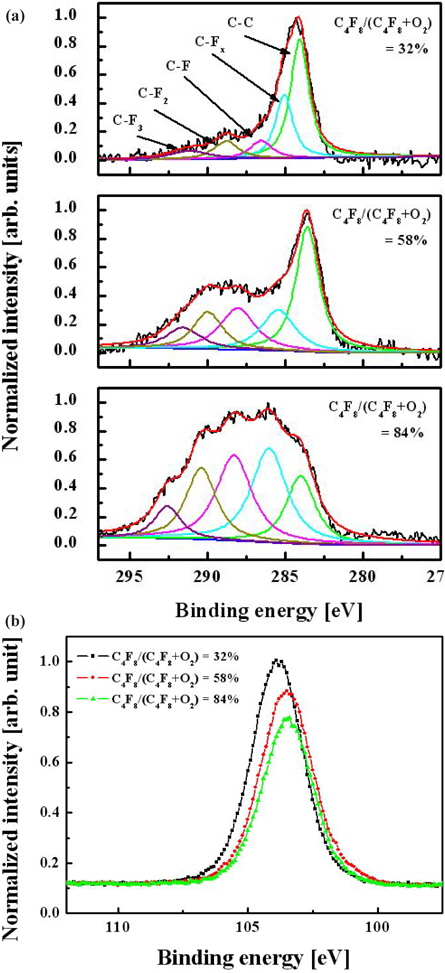
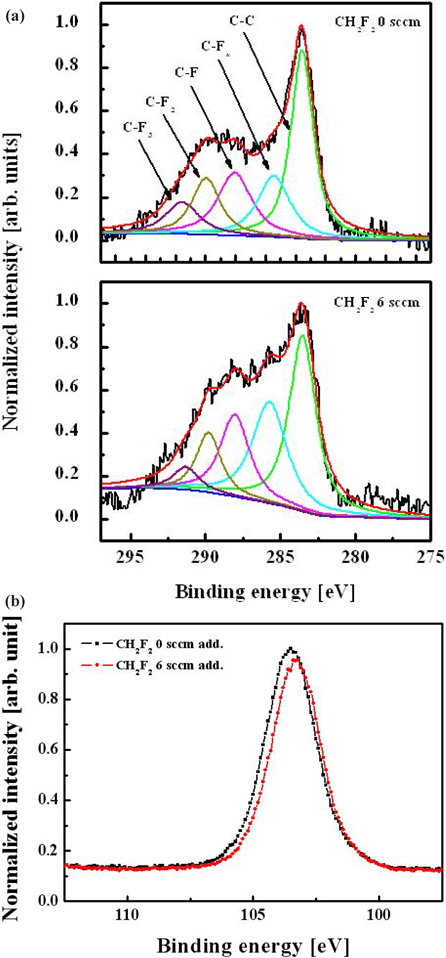
Figure 4 shows C 1 s and Si 2 p XPS narrow-scan spectra for surfaces etched with various gas mixing ratios. A wide C 1s peak can be deconvoluted into five peaks according to well-known data on binding energies for carbon-containing compounds. These peaks are 284.3 eV (C-C), 286.6 eV (C-CFx), 288.8 eV (C-F), 290.1 eV (C-F2), and 293.2 eV (C-F3) [9, 10]. Each spectrum shown in Fig. 4(a) shows a similar qualitative composition, except for peak intensities. As the C4F8/O2 ratio increases, the CF2 and CF3 bonding increases significantly and their intensities reach the same value as the C-C and C-F peaks. This indicates a growth of the CFx polymer layer, which has a fluorine-rich and low-carbon composition at high C4F8 contents. Figure 4(b) shows that the Si 2 p narrow-scan spectra cannot be resolved in the SiO (102.7 eV), SiO2 (103.6 eV), and SiC (100.7 eV) compounds due to their adjacent binding energies. However, as the C4F8/O2 gas mixing ratio increases, the intensity of Si 2 p narrow-scan spectra decreases because the increased CFx radical obstructs the reaction between the O species and silicon. This phenomenon was related to the change of the etch property.
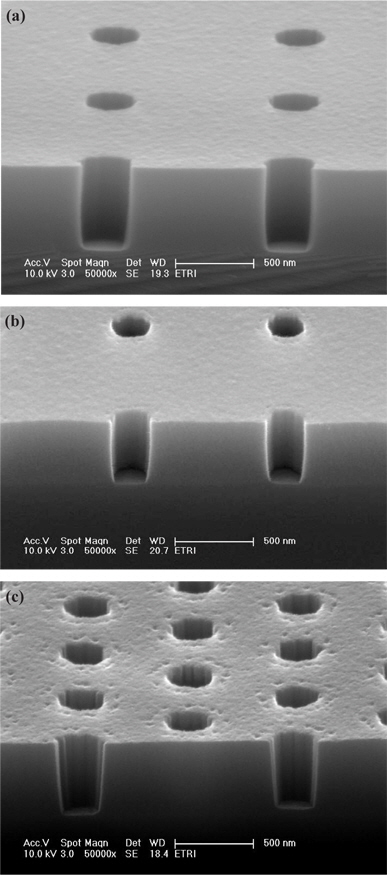
Figure 5 shows the cross-sectional SEM image of the etched contact holes pattern as a function of the C4F8/O2 mixing ratio in the C4F8/O2/Ar plasma. The profile of holes is close to 90° in Fig. 5(a), and the etch residue is not found at the sidewall. As the C4F8 fractional pressure increases, the oxide etch rate and selectivity of oxide to PR increases, but it has a disadvantage at the etch profile.
Although an adequate etch profile was obtained, a higher etch selectivity is required to make self-aligned contacts and to provide deeper etching. To find the process condition that provides better etch selectivity, we investigated the effect of CH2F2 addition in C4F8/O2/Ar (C4F8/O2/Ar=18/13/300) plasma.
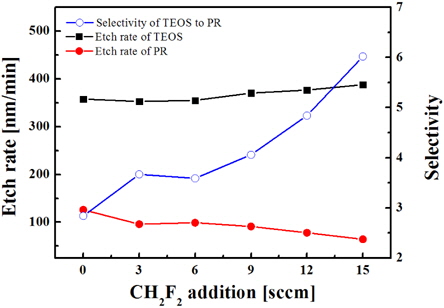
Figure 6 shows that the addition of CH2F2 results in increasing the etch rate of SiO2, but in decreasing the etch rate of PR, so that the etch selectivity of oxide over PR increases. These effects may be associated with the role of hydrogen atoms formed in plasma due to the dissociation of CH2F2, and at least two mechanisms can be proposed. First, H atoms can attach to atomic fluorine to form HF molecules. This results in decreasing the volume density of F atoms, decreasing the recombination rate of F with CFx, and thus increasing the density of CFx, which is the main chemically active species for oxide. This mechanism is in good agreement with the OES data shown in Fig. 7.
From Fig. 7, it can be seen that the addition of CH2F2 is accompanied by an increase in the emission intensity of the CF2 band that can be associated with increasing volume density for these species. Second, hydrogen can assist the chemical etching of oxide by extracting oxygen atoms to form OH radicals.
Figure 8 shows the C 1s and Si 2 p XPS narrow-scan spectra of the etched oxide surface with and without an admixture of CH2F2 in the C4F8/O2/Ar plasma. The addition of CH2F2 increases the density of the CFx bonds on the surface, which can be associated with the increasing flux of these species coming from bulk plasma. The intensity of the Si 2 p XPS narrow-scan spectra is shown in Fig. 8(b). This peak can comprise SiC, SiO, and SiO2. As mentioned above, in Fig. 4, the intensity change of this peak affects the etch property. Therefore, Fig. 8 demonstrates the increasing volume density of CFx radicals (as previously mentioned with the OES measurements) and confirms the mechanism proposed to explain the acceleration of the oxide etch rate in the presence of CH2F2 molecules.
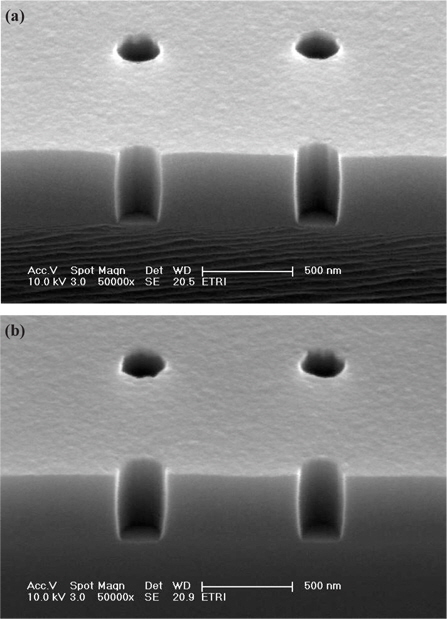
Figure 9 shows the cross-sectional SEM image of the etched contact holes pattern as a function of CH2F2 addition in the C4F8/O2/Ar plasma. When the CH2F2 gas was added, a slight bowing profile was shown, but higher selectivity of oxide to PR could be obtained. Moreover, the bowing profile can be removed by changing the gas mixture. However, the excessive CH2F2 gas addition induces sidewall roughness of the contact hole, as confirmed in Fig. 9(b).
In this work, we investigated the etching of contact holes through a PR mask using C4F8/O2/Ar and CH2F2/C4F8/O2/Ar gas mixtures. As the mixing ratio of C4F8 on the C4F8/O2 increases, the amount of polymer deposited on the etched surface also increases. This leads to an increase in the etch selectivity of oxide over PR because the CxFy polymer layer retards the reaction of oxygen atoms with PR. The profile of the contact holes was found to be close to 90°, and the etch residue is not found at the sidewall at a C4F8/(C4F8+O2) ratio of 58%. We also found that the etch selectivity of oxide over PR can be improved by the addition of CH2F2 in the C4F8/O2/Ar plasma because the CFx radical volume density is increased.