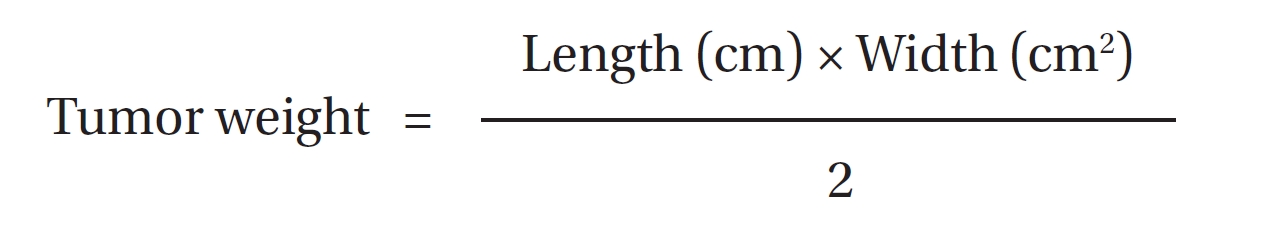Breast cancer is one of the leading causes of death worldwide. Breast cancer statistics show the need for new methods to control this deadly form of cancer [1]. Free radical and non-radical oxidizing species are frequently produced in animals treated with carcinogens, and mounting evidence suggests that these free radicals and electrophile mediated oxidative stress play important roles in all stages of chemical carcinogenesis and tumorigenesis [2]. 7,12-dimethyl benz[a]anthracene (DMBA) is a well recognized carcinogen known to induce enormous amounts of free radicals, which in turn reacts with lipids, causing the release of lipid peroxides [3].
Epidemiological data have indicated favorable effects of antioxidant compounds in the prevention of a multitude of diseases, including cancer, cardiovascular diseases and neuro degenerative diseases [4]. Taurine is one such compound that acts as an antioxidant and prevents oxidative stress [5-8]. Taurine (2-amino ethane sulphonic acid) is a sulfur-containing semi-essential amino acid that is, endogenously synthesized and is provided by the diet, especially by fish and meat. Several studies have reported that taurine acts as an antioxidant and prevents oxidative stress [9-10]. The role of taurine on mitochondrial function and anti-cancer activity also has been studied [11-12].
Therefore, the primary object of the present study is to reveal the beneficial role of taurine in modifying the hepatic mitochondrial enzyme system with respect to lipid peroxidation (LPO), antioxidant status, major citric acid cycle enzymes, and electron transport chain complexes during DMBA-induced mammary cancer in rats.
DMBA and taurine were purchased from Sigma chemicals (St. Louis, U.S.A.). All other chemicals were of analytical grade and were procured from SRL Chemicals Pvt. Ltd., Mumbai, India.
The animals used in this experiment were healthy female Sprague Dawley rats, 6 ─ 8 weeks of age and weighing about 150 ─ 180 g. The animals were procured from Central Animal House Facility, Dr. ALM PG IBMS, University of Madras, Taramani, Chennai 600-113, India. The rats were acclimatized to laboratory conditions with a 12-hours light/dark cycle under constant temperature and humidity and were given ad- libitum access to a balanced diet (Gold Mohor rat feed, M/s. Hindustan Lever Ltd., Mumbai). This research was approved by The Institutional Animal Ethical Committee (IAEC no.01/19/2012).
Experimental animals were divided into four groups of six rats each as follows:
Group I: Contained the normal control animals. These were fed with the standard diet and pure drinking water.
Group II: Contained the animals induced with a single dose of DMBA (25 mg/kg body-weight (b-wt)) in 1.0 mL olive oil by gastric intubation to induce breast cancer [13, 14]. These animals received no further treatment.
Group III: Contained breast cancer bearing animals that were post treated with taurine (100 mg/kg b-wt in pure drinking water by gastric intubation) once daily, starting ten weeks after the DMBA administration and continued upto and including the 15th week.
Group IV: Contained the control animals treated with taurine alone from the 10th up to and including the 15th week.
At the end of the experimental period, animals were sacrificed by cervical decapitation under ether anesthesia, and breast tissues were excised immediately and washed with ice cold saline. A 10% homogenate of the washed tissue (breast) was prepared in 0.01-M phosphate buffer (pH 7.4). The homogenate was centrifuged at a speed of 12,000 × g for 30 minutes in a refrigerated high speed centrifuge at 4℃. Blood was also collected, and the serum was separated for other analyses. The following biochemical analyses were carried out in the supernatant and in the serum.
The mitochondria of breast tissues were isolated by the method of Johnson and Lardy [15]. A 10% (w/v) homogenate was prepared in 0.05 M Tris-hydrogen chloride (HCl) buffer, pH 7.4, containing 0.25-M sucrose and was centrifuged at 600 × g for 10 minutes. The supernatant fraction was decanted and centrifuged at 15,000 × g for 5 minutes. The resultant mitochondrial pellet was then washed and re-suspended in the same buffer.
The post mitochondrial fraction was further centrifuged at 105,000 × g for 60 minutes. The supernatant was cytosol and the pellet was microsomal. The microsomal pellet was suspended in 0.05-M Tris-HCl buffer, pH 7.5, containing 0.15-M potassium chloride (KCl). The purity of mitochondrial fraction was assessed by measuring the activity of succinate dehydrogenase.
The protein was estimated by using the method of Lowry
The tumor weights were estimated [31]. The resultant solid tumor was considered to be prelate ellipsoids with one long axis and two short axis of the same lengths. The two short axes were measured with a vernier caliper. The tumor weight was calculated by multiplying the length (long axis) of the tumor by the square of the width (short axes) and dividing the product by 2.
All data were expressed as the mean ± standard deviation (S.D) for six rats. The results were computed statistically (SPSS Software Package) by using the one-way analysis of variation (ANOVA). Post-hoc testing was performed for inter comparisons by using the least significant difference (LSD). (
(Table 1) shows the tumor weights in breast cancer bearing animals not treated with taurine (Group II) and in the breast cancer bearing animals treated with taurine (Group III). The tumor weights were significantly (
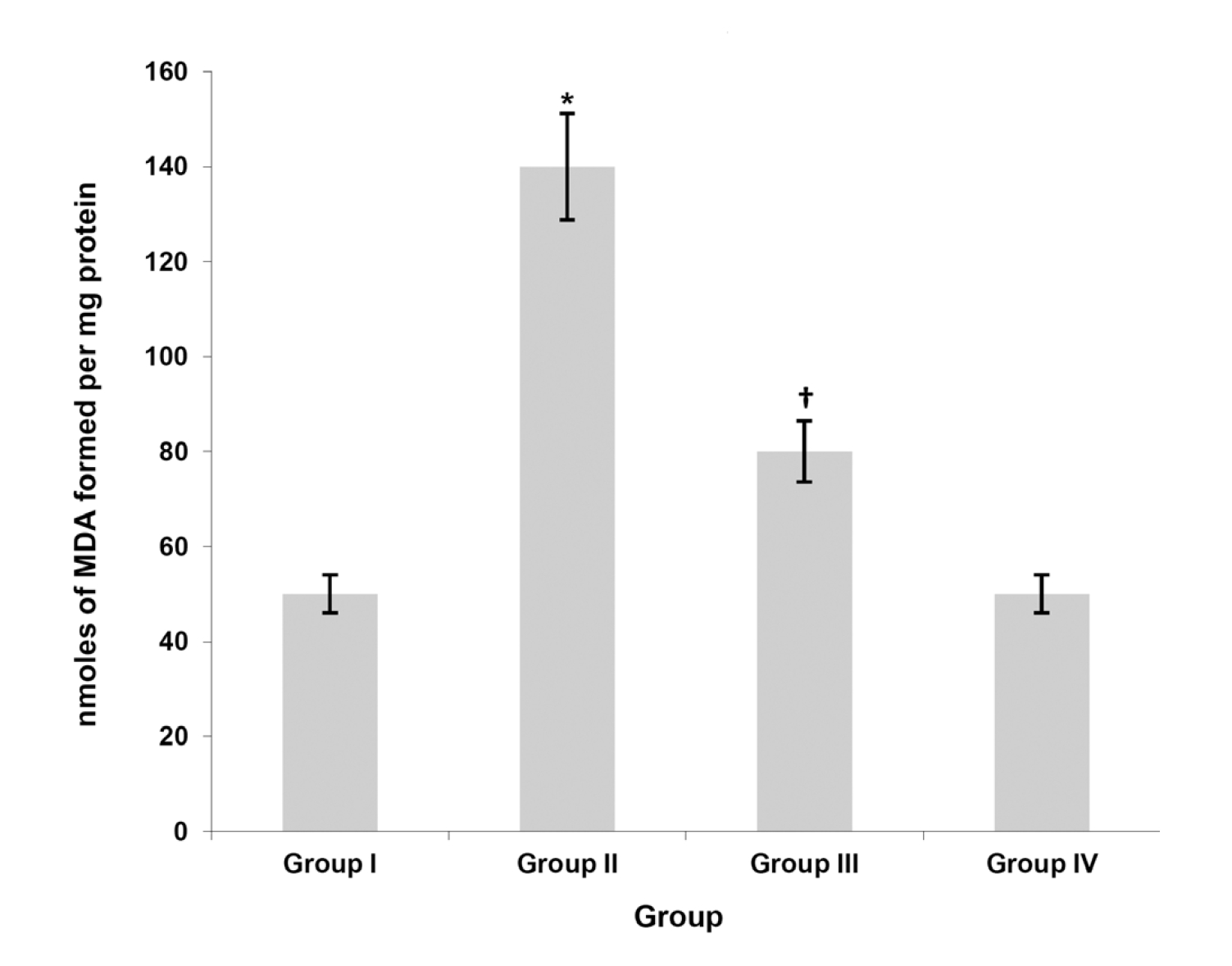
(Fig. 1) shows the effect of taurine on liver LPO levels. DMBA-induced breast cancer bearing Group II animals showed significantly (
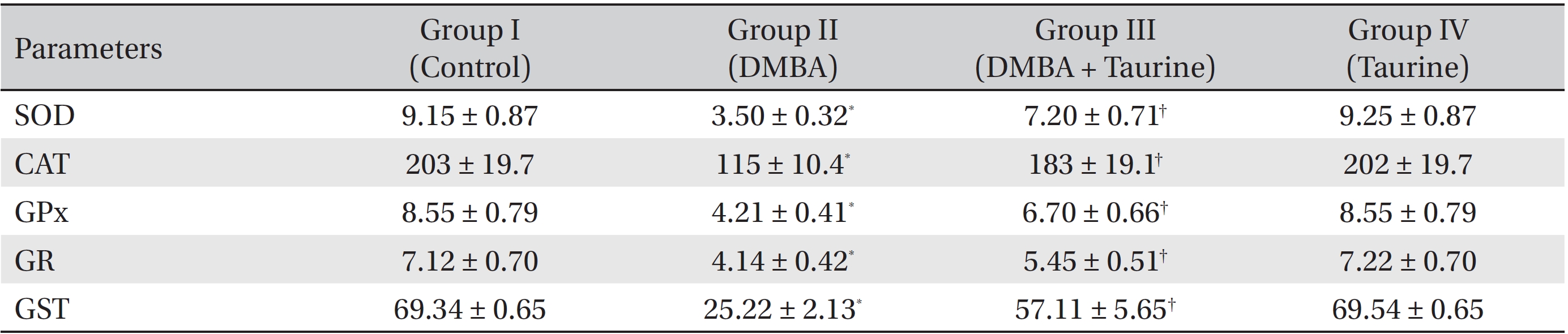
(Table 2) depicts the effect of taurine on liver enzymic antioxidant status. Significant (
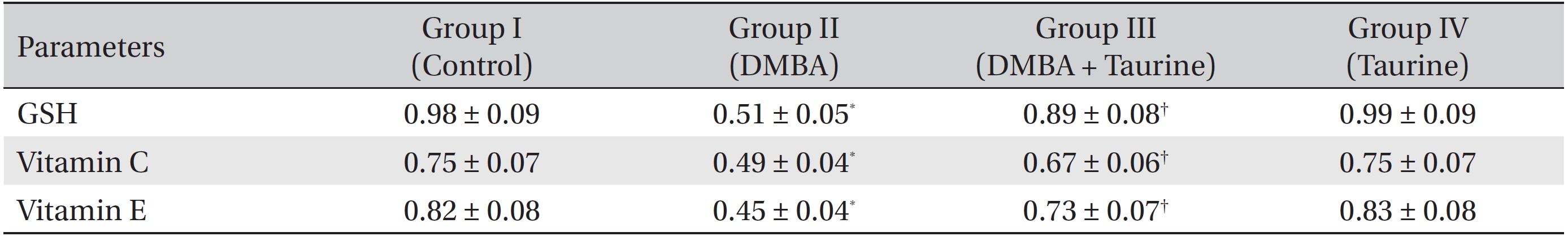
(Table 3) shows the levels of the non-enzymic antioxidants GSH, vitamin C and vitamin E in the control and experimental groups of animals. Animals with DMBA-induced breast cancer (Group II) showed markedly (
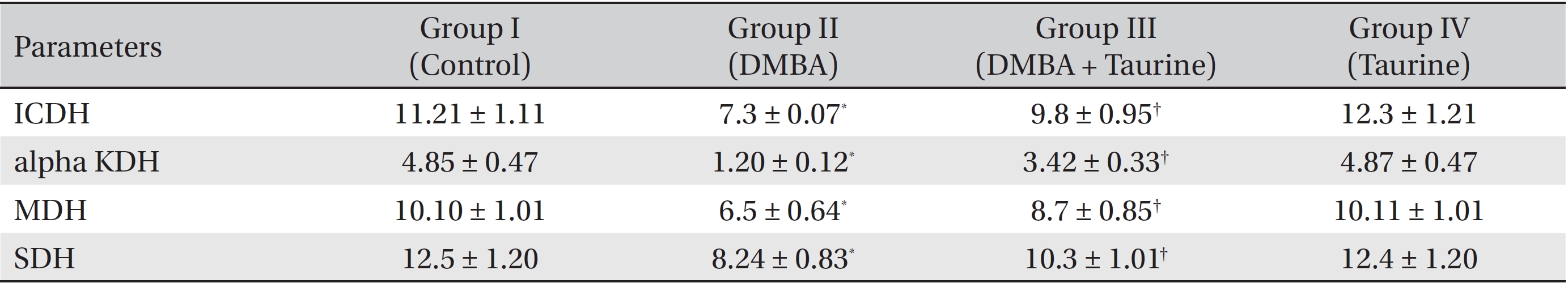
The influence of taurine on the activities of key citric acid cycle enzymes is shown in (Table 4). The activities of the citric acid cycle enzymes ICDH, alpha KDH, MDH, and SDH were found to be significantly (
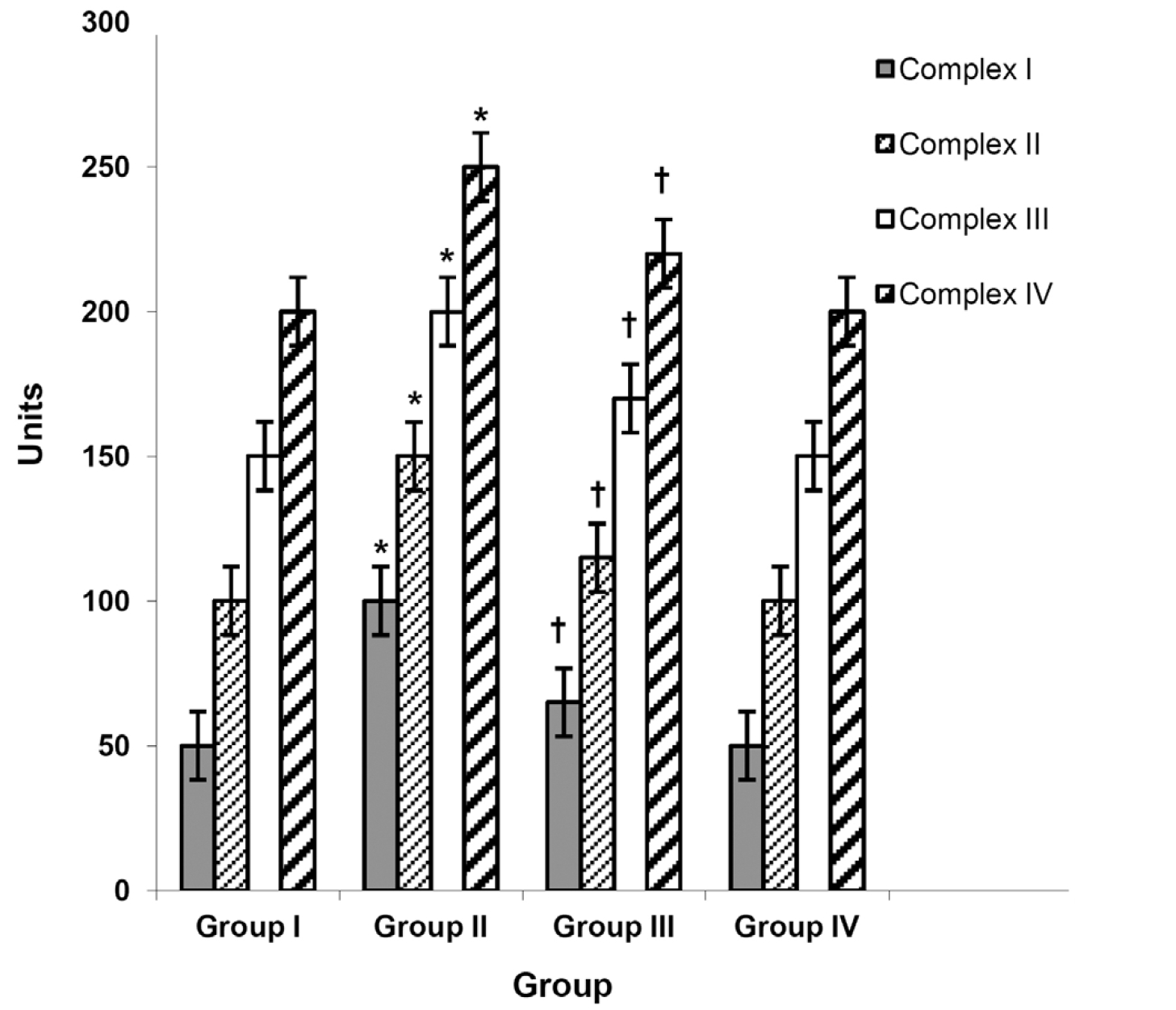
(Fig. 2) shows the activities of liver ETC complexes in the control and the experimental groups of animals. In breast cancer bearing animals (Group II), activities of ETC complex enzyme were significantly (
Progressive cellular architectural changes due to oxidative stress, LPO and modulation of various cellular molecular pathways by reactive free radicals generated during the metabolism of DMBA have been implicated in the pathogenesis of mammary carcinogenesis [32]. The mitochondrion is now gaining importance in cancer research because of its central role as a regulator of energy balance, and the mitochondrion appears to be the primary target for oxidative stress induced damage during cancer as it has been suggested to be the main source of free radical production [33]. Our results were in agreement with the above findings in that we observed an increase in mitochondrial LPO in animals with DMBA-induced breast cancer.
Identification of chemopreventive agents, which can either abolish or delay the development of carcinogenesis, has become an ideal strategic paradigm in the struggle against cancer. The main mechanism accounting for the abilities of these substances to combat the reactive oxygen species (ROS) induced oxidative damage is their antioxidant effect [34]. Taurine is one such compound whose potential antioxidant role has been reported [7]. The above findings were substantiated by our present study, in that treatment with taurine reduced mitochondrial LPO induced by DMBA, suggesting the antioxidant potential of taurine.
Induction of antioxidant enzymes is a major approach for protecting cells against a variety of endogenous and exogenous toxic compounds such as ROS and chemical carcinogens [35]. SOD, which converts superoxide radicals to hydrogen peroxide, is widely distributed in cells having oxidative metabolism and is thought to protect such cells against the toxic effects of the superoxide anion [36]. CAT is a heme protein that catalyzes the direct degradation of hydrogen peroxide to water. It protects the cellular constituents against oxidative damage [37]. GPx catalyzes the reduction of hydrogen peroxide and hydroperoxide to non-toxic products and scavenges the highly reactive lipid peroxides in the aqueous phase of cell membranes [38]. GST is a group of multifunctional proteins that perform tasks ranging from catalyzing the detoxification of electrophilic compounds to protection against peroxidative damage [39]. GR plays a major role in regenerating GSH from glutathione disulfide (GSSG), thus maintaining the balance between the redox couple [40].
The activities of the mitochondrial antioxidant enzymes SOD, CAT, GPx, GST, and GR were observed to be lowered in the DMBA-induced cancer bearing animals. On taurine supplementation, the activities of these enzymes were markedly elevated in Group III animals. This may be due to the direct reaction of taurine with superoxide, hydroxyl and alkoxyl radicals, which in turn, reduces free radical formation and mitochondrial oxidative damage during mammary cancer [9-10].
Reduced glutathione plays a vital role in the detoxification of xenobiotic compounds, and in the antioxidation of ROS and free radicals [41]. Decreased GSH levels signify increased oxidative stress. In our present study, we observed a decline in GSH levels in cancer bearing animals that had not been treated with taurine (Group II), which may have been due to the excess utilization of this antioxidant for tumor cell proliferation. Taurine treatment of cancer bearing animals (Group III) elevated GSH levels. Vitamin C, an important antioxidant, acts in tissues, involving ROS in an aqueous phase, and the tissue concentration of vitamin C has been reported to be a good indicator of oxidative stress [42]. Vitamin E is a principal lipid soluble antioxidant in cell membranes and protects critical cellular structures against oxidative damage [43]. Vitamin C and E levels were found to be reduced in breast cancer bearing animals, suggesting an increase in LPO in these animals. The levels of these vitamins in rats challenged with DMBA were almost normalized upon taurine treatment (Group III).
The major enzymes of the citric acid cycle, ICDH, alpha KDH, MDH, and SDH, are involved in the maintenance of the reduced redox state in mitochondria in order to provide the reducing power to generate adenosine triphosphate (ATP) via oxidative phosphorylation. In the present study, significant decreases in these citric acid cycle enzymes were observed in animals with breast cancer that had been induced by using DMBA (Group II). This reduction in the activities of these enzymes might be due to alterations in the morphologies of the cancer cells, ultra structural changes, and the ability of mitochondria to undergo metabolic changes. Taurine treatment regulated the activities of these mitochondrial enzymes at levels close to near normalcy, revealing its chemoprotective nature (Group III).
The ATP essential for cellular activity is almost exclusively mitochondrial in origin. This ATP production depends upon the proper functioning of the mitochondrial ETC and of the functionally coupled proton motive ATP-synthase (ATP-ase). nicotinamide adenine dinucleotide (NADH)- ubiquinone oxidoreductase, also known as Complex I, is a multi subunit integral membrane complex of the mitochondrial ETC which catalyzes electron transfer from NADH to ubiquinone. Cytochrome bc1 complex (Complex III) is considered to be crucial for the activity of the entire respiratory chain and appears to be well coupled with succinate dehydrogenase (Complex II). Cytochrome c oxidase (Complex IV) is the terminal enzyme of the mitochondrial respiratory chain, catalyzing the reduction of molecular oxygen with electrons from reduced cytochrome c and concomitantly conserving the reaction energy by pumping protons across the inner mitochondrial membrane. Oxidative stress mediated LPO has been reported to affect the lipid environment of the membrane, thus affecting the activity of these ETC enzymes in DMBA-induced cancer bearing animals [44]. Decreases in the activities of ETC complexes may, in turn, promote the leakage of electrons from the mitochondrial inner membrane associated with electron transport complexes contributing to increased mitochondrial ROS generation [45]. Our results are in accordance with the above findings as we have observed a significant decline in the activities of ETC complexes in DMBA-induced cancer bearing animals (Group II). Taurine treatment inhibited LPO, leading to a marked increase in the activities of these complexes (Group III).
The results of our present investigation demonstrate that taurine supplementation alleviated the alterations in mitochondria induced by DMBA and regulated the mitochondrial function during DMBA-induced mammary carcinogenesis.
[Table. 1] Effect of taurine on breast tumor weight of the control and the experimental animals

Effect of taurine on breast tumor weight of the control and the experimental animals
[Table. 2] Effect of DMBA and taurine on enzymic antioxidants in liver mitochondria
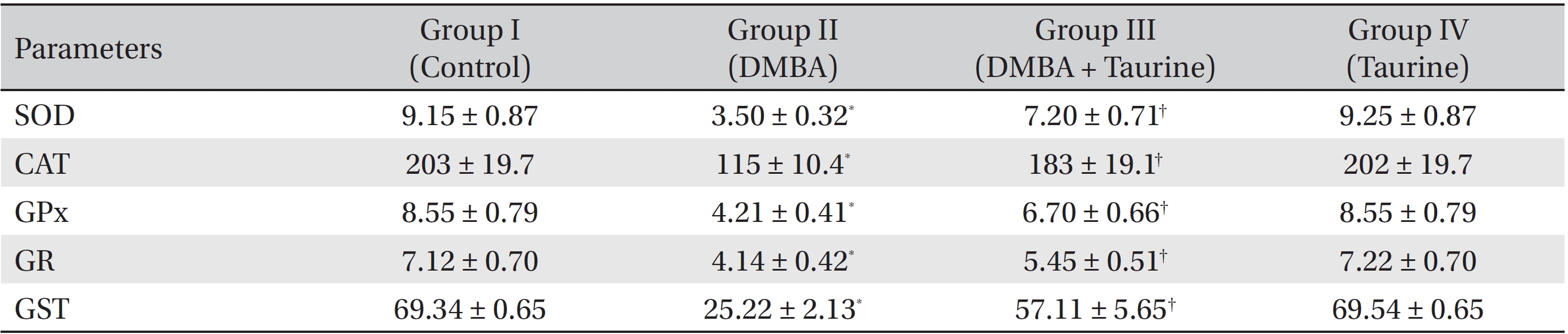
Effect of DMBA and taurine on enzymic antioxidants in liver mitochondria
[Table. 3] Effect of DMBA and taurine on non-enzymic antioxidants in liver mitochondria
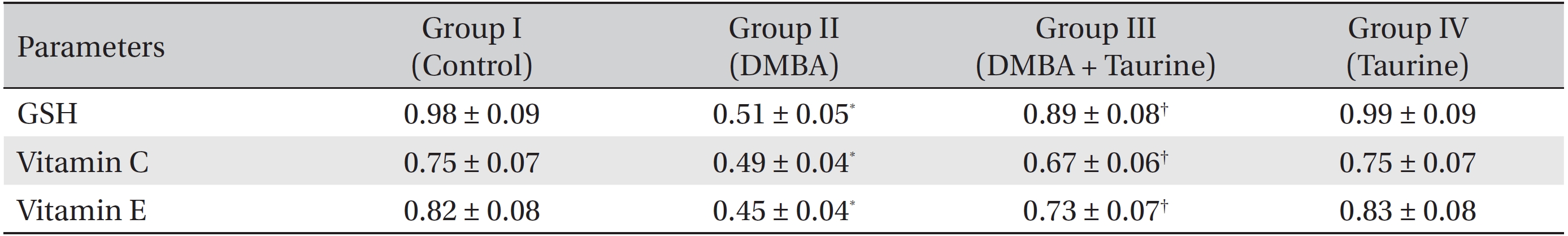
Effect of DMBA and taurine on non-enzymic antioxidants in liver mitochondria
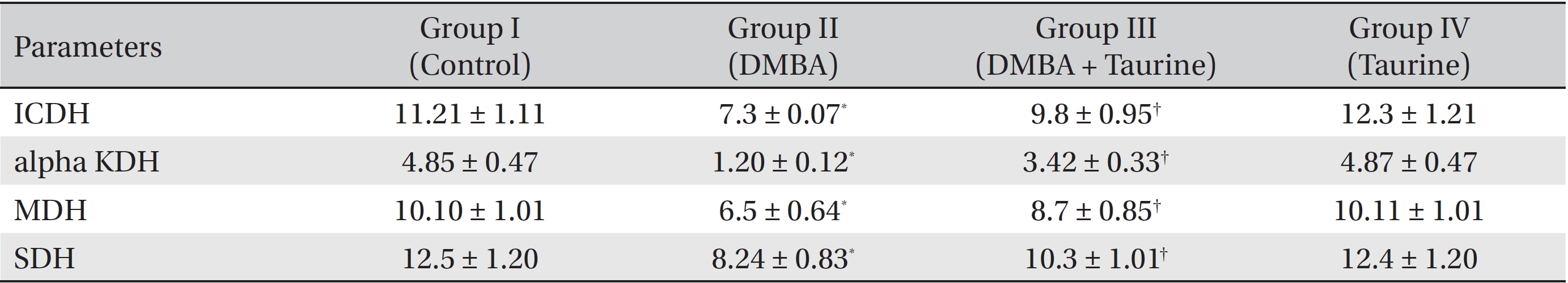
Effect of DMBA and taurine on the activities of major citric acid cycle enzymes in liver mitochondria