Correlations of the levels of the nonspecific inflammatory markers C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) and of the coagulation marker fibrinogen with the treatment period of wheel balanced cancer therapy were determined.
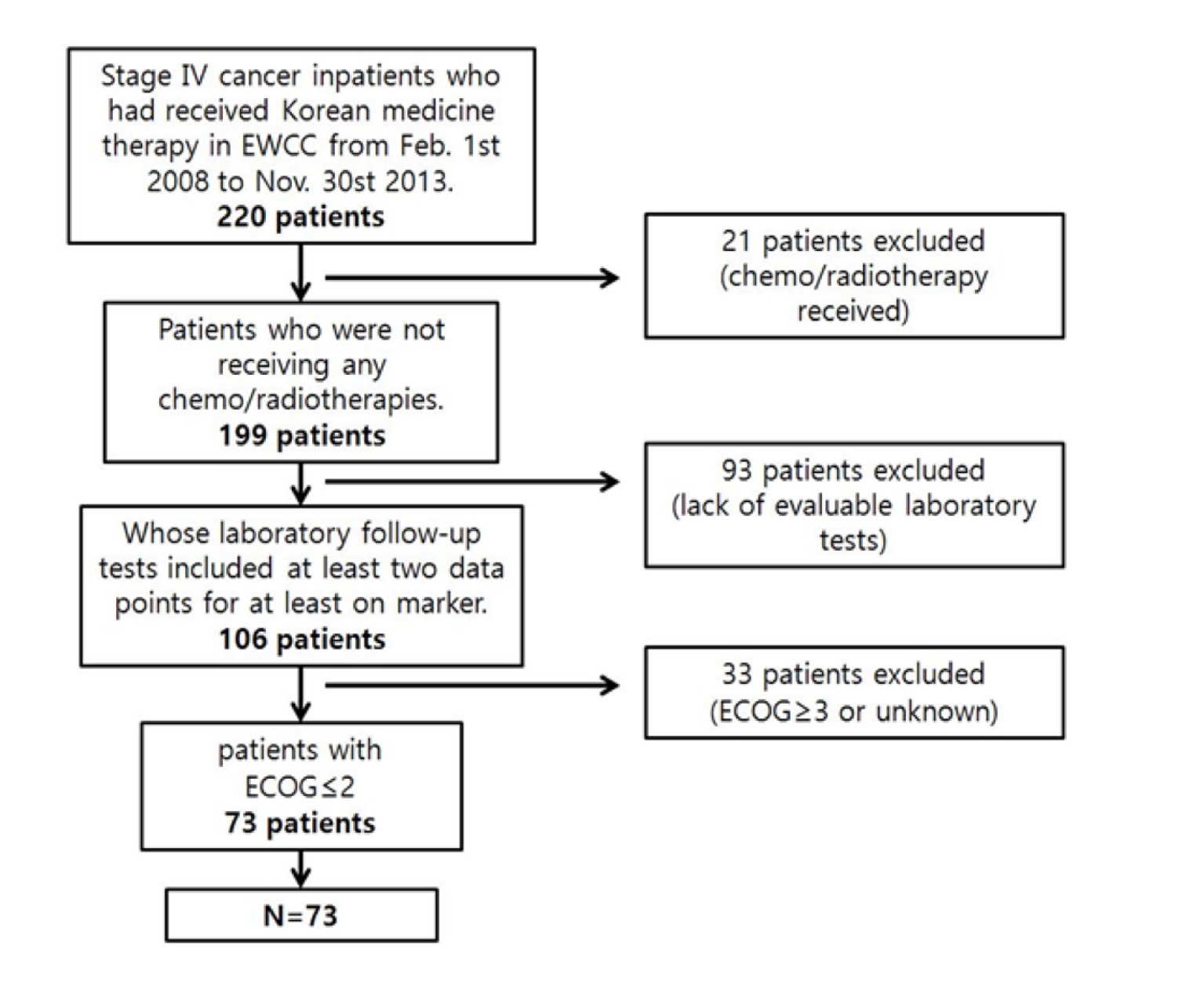
Electronic charts of stage IV cancer patients hospitalized from February 1, 2008, to November 30, 2013, were reviewed retrospectively. Patients whose laboratory follow-up tests included at least two data points for at least one marker were included. Patients receiving chemotherapy or radiotherapy or having Eastern Cooperative Oncology Group (ECOG) levels exceeding 2 were excluded. Correlations of the markers with the length of treatment for treatment periods ≥ 21 and ≤ 20 days were determined by gender and whether or not surgery had been performed.
Analyses of the CRP and the ESR revealed a higher proportion of patients with stable marker levels than with increased or decreased levels. Also, only the ESR in female and the CRP in male groups had higher proportions of patients with stable marker levels than with increased or decreased levels. The ≥ 21 day group had a higher proportion of patients with stable CRP and ESR levels than the ≤ 20 day group. Only the ESR in female and the CRP in male groups had higher proportions of patients with stable marker levels in the ≥ 21 day than in the ≤ 20 day group. In addition, only the CRP in the surgery group and the ESR in the non-surgery group had higher proportions of patients with stable marker levels in the ≥ 21 day group than in the ≤ 20 day group.
For stage IV cancer patients at hospitals that offer Korean medicine, more than 21 days of long-term wheel balanced cancer therapy (WBCT) should help maintain the CRP and the ESR levels and should have a favorable effect on the survival rate.
C-reactive protein (CRP), which is synthesized by hepatocytes, is elevated under acute phase inflammatory conditions and plays an important role in the immune system [1]. CRP is also elevated in various neoplastic diseases and is considered as a prognostic factor in advanced cancer patients [2]. An elevated serum CRP level was found to be significantly related to the incidence of liver metastases, peritonitis carcinomatosa, pathological lymph node metastases, intravenous invasion, and survival rate in 120 colorectal cancer patients [3].
The erythrocyte sedimentation rate (ESR) is a nonspecific inflammatory marker that responds to systemic inflammation [4, 5]. In a study of 889 clear cell renal cell carcinoma patients, patients with elevated ESR showed a significant unfavorable association with tumor thrombus, large tumor size, advanced stage, lymph node, and distant metastasis in comparison with patients whose ESR was within normal limits [6]. Elevated ESR is also associated with poor survival rates in prostate cancer patients and non-small cell cancer (NSCLC) patients [7, 8].
Fibrinogen is synthesized by hepatocytes and is necessary for the production of fibrin sheaths to coagulate blood during a hemorrhage [9]. In a study of fibrinogen deficient mice with implanted lung cancer cell, fibrinogen was proven to function in tumor metastasis, adhesion, and survival; thus, the serum level of fibrinogen is considered as an indicator of the metastatic potential [10].
The above studies indicate that elevated CRP, ESR, and fibrinogen are poor prognostic factors for tumor progression, tumor size, tumor depth of invasion, postoperative residual mass, and survival rate for advanced cancer patients. In the treatment of advanced and refractory cancer patients, reducing the size of the tumor might be less crucial than maintaining the state of the tumor over time. According to a meta analysis of the association between the use of herbal medicine and survival, herbal medicine combined with chemotherapy resulted in favorable survival rates at 12, 24, and 36 months compared to chemotherapy alone [11].
Many studies report that Chinese herbal medicine lowers blood viscosity and removes heat from blood. Liangxuehuayu decoction has both the effects of accelerating blood to remove stasis and cooling blood in rats by reducing the fibrinogen level and platelet aggregation and by prolonging the prothrombin time [12]. The Taoren Honghua herb pair promotes blood circulation, lowers whole blood and plasma viscosity, prolongs the thrombin time and lowers the fibrinogen level by influencing hemorheology [13]. A group of cancer patients who received Korean medicine therapy has been reported to have shown a larger decrease in the mean fibrinogen level and natural killer (NK) cell concentration than the prevention or the conventional combination therapy groups did, suggesting a possible effect of Korean medicine on tumor angiogenesis, malignant transformation, and metastasis, which involve signaling cascades triggered by tissue factor [14]. However, the above studies have mainly analyzed the levels of fibrinogen or NK cells without assessing the CRP or the ESR levels. Thus, we decided to carry out this study of the correlation between the treatment period of wheel balanced cancer therapy (WBCT), which is a cancer care program of the East West Cancer Center (EWCC) and the CRP, ESR and fibrinogen levels associated with tumor progression, metastasis, and survival rates.
2.1. Patient eligibility (Patients)
A retrospective electronic chart review was conducted for 220 cancer patients. Data were from a single site and were collected under a protocol approved by the Daejeon University Institutional Review Boards (IRB, IRB approval number: 14-09). StageⅣcancer patients hospitalized at the EWCC for Korean medicine therapy alone from February 1, 2008, to November 30, 2013, were included in this study. Patients who had undergone chemotherapy or radiotherapy during the treatment period were excluded as were patients whose laboratory follow-up tests did not include at least two data points for at least one marker (CRP, ESR, or fibrinogen), patients whose Eastern Cooperative Oncology Group (ECOG) grade was 3 or more, and patients with an unknown ECOG grade (Fig. 1).
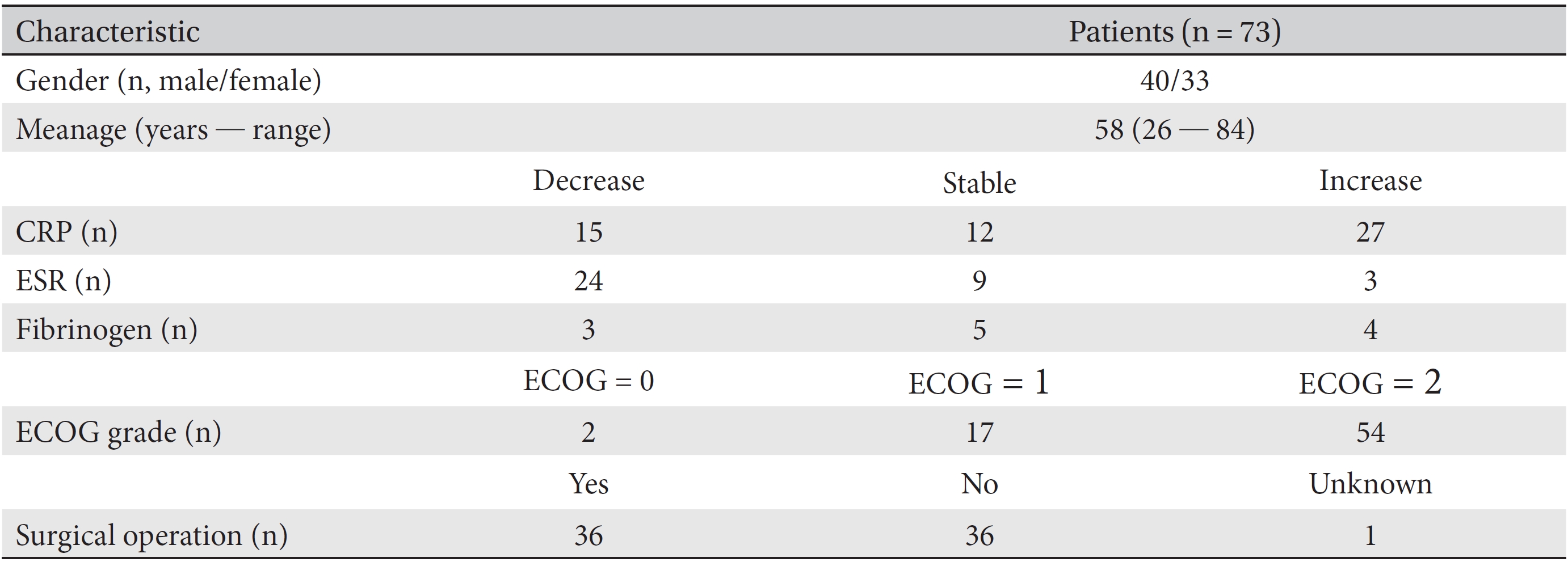
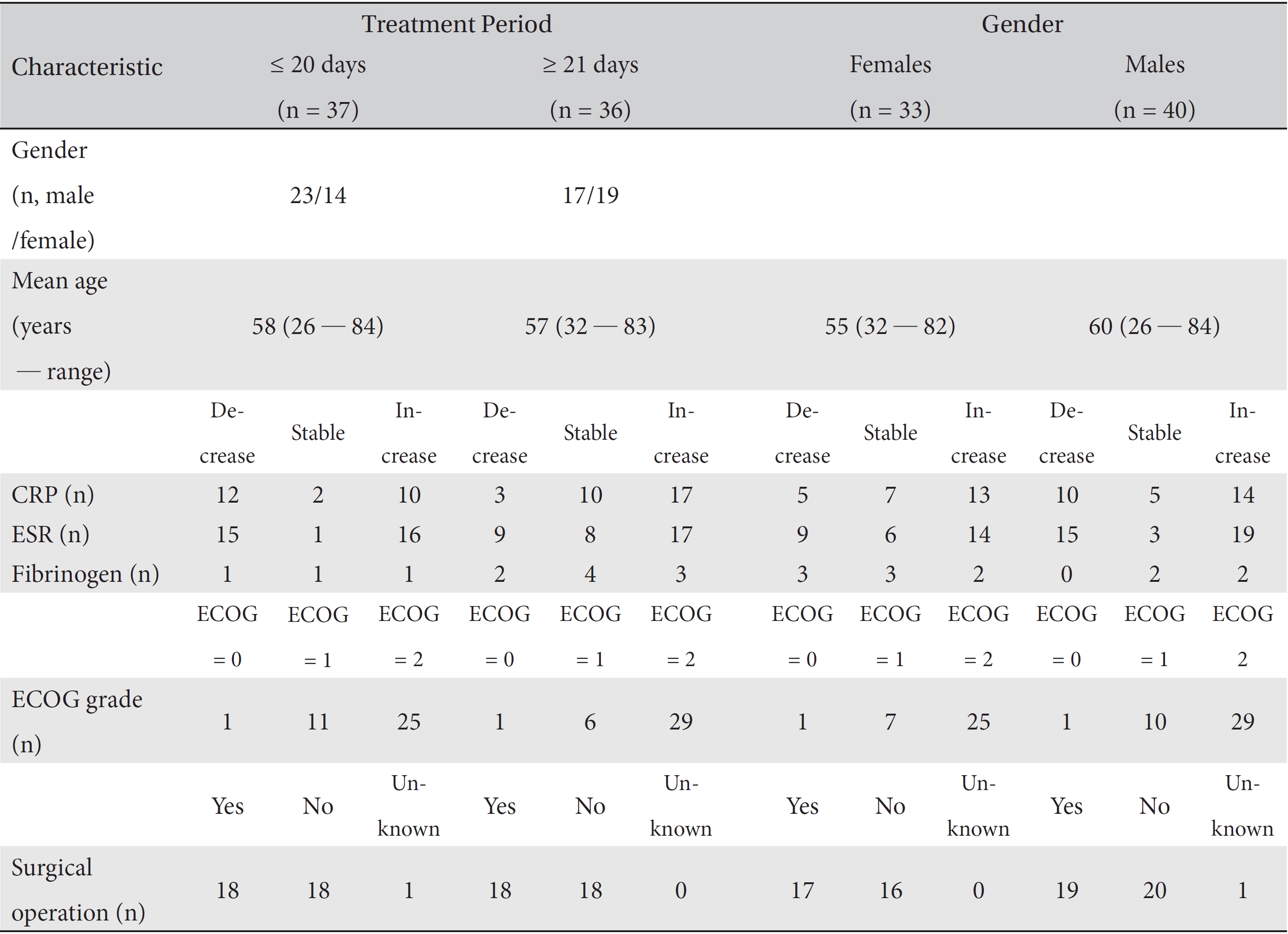
Finally, 73 patients were included. All data from CRP, ESR and fibrinogen test results, along with the patients ages, genders, ECOG grades and surgical operations, throughout their treatment period were collected. Then, the necessary data from the first and the last test results for the markers were collected. All data were collected after de-identification of the patient by using a unique review number. Information on the variations in the patients CRP, ESR, and fibrinogen levels was analyzed in separate groups according to the treatment period, past surgery, and gender. The clinical characteristics of all 73 patients are described in (Tables 1,2,3).
2.2. Wheel balanced cancer therapy
WBCT, the multi modality cancer program for EWCC's inpatients, which includes the following four sub programs, is provided daily to all inpatients of the center [14]: First, herbal therapy composed of anti-angiogenic, immune activating agents and herbal decoctions prescribed according to the Korean medicine differential diagnosis is provided daily. Second, a cancer prevention diet composed of constitution specific foods is provided. Vegetables or fruit juices that have anti-oxidative activity are provided once or twice a day. Third, metabolism activation, which is composed of acupuncture, pharmacopuncture, massage, moxibustion, hydrotherapy, and thermotherapy, is provided to inpatients. Fourth, mind body therapy, which is composed of muscle relaxation therapy, meditation and yoga, and hiking, is provided.
The decrease state was defined as a decrease from the abnormally increased state to a normal level or at least a 5% reduction from the first level. The increase state was defined as an increase from the normal level to the abnormally increased state or at least a 5% increase from the first level. The stable state was defined as the state in which the values remained within the normal level or experienced a < 5% variation within the abnormal level. Conditions not included in these categories were considered as unknown. The laboratory follow-up data were checked before and after treatment.
The data for the marker levels, which were classified as decreased, increased and stable levels, were analyzed according to the length of the treatment period. First, a oneway analysis of the correlations between the CRP, ESR, and fibrinogen levels of the 73 patients and the length of the treatment period was conducted. Second, a one-way analysis of the correlations between the marker levels and the length of the treatment period was conducted for both male and female subgroups. All one-way analyses were conducted by using the Kruskal-Wallis test.
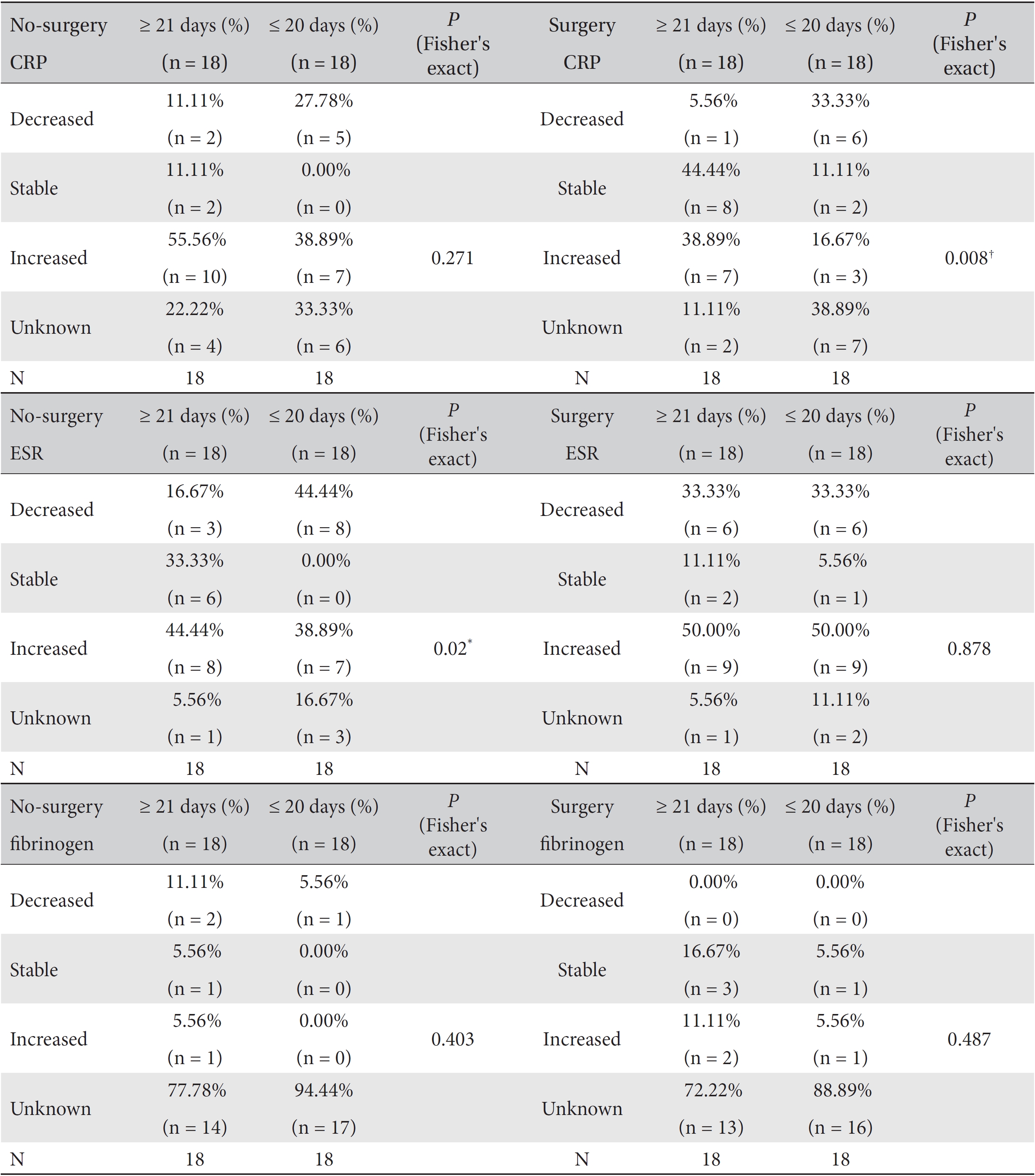
Cross analyses of the correlations of the marker levels to the lengths of the treatment periods for both the male and the female subgroups and for the surgery and the non-surgery subgroups were conducted. First, a cross analysis between the marker levels and the length of the treatment period divided into ≤ 20 day and ≥ 21 day groups (days refer to the length of the treatment period) was conducted without subgroups because the mean treatment period of the stage Ⅳ cancer inpatients was 21 days. Second, a cross analysis between the marker levels and the length of the treatment period divided into ≤ 20 day and ≥ 21 day groups was conducted for both the male and the female subgroups. Third, a cross analysis between the marker levels and the length of the treatment period divided into ≤ 20 day and ≥ 21 day groups was conducted for both the surgery and the non-surgery subgroups. Additionally, a cross analysis of the correlation between the marker levels and surgery was conducted. All cross analyses were conducted by using Fisher's exact test and the likelihood ratio test. Then, the proportions of decreased, increased and stable level during the treatment period were compared between the ≤ 20 day and the ≥ 21 day groups.
For fibrinogen, the study population was so small that it did not meet the normality assumption required by the Shapiro-Wilk and Kruskal-Wallis tests. In the case of the CRP and the ESR, the study populations were large enough for the tests, but they also did not meet the normality assumption required by the Shapiro-Wilk test. Thus, variations in the CRP and the ESR levels were analyzed by using the Kruskal-Wallis test.
Because all three markers did not meet the normality assumption required by the Shapiro-Wilk test, the nonparametric Kruskal-Wallis test was used for the one-way analyses. For cross analyses of these markers, Fisher's exact test and the likelihood ratio test were used together.
3.1. One-way analyses of the correlations between the markers and the treatment period
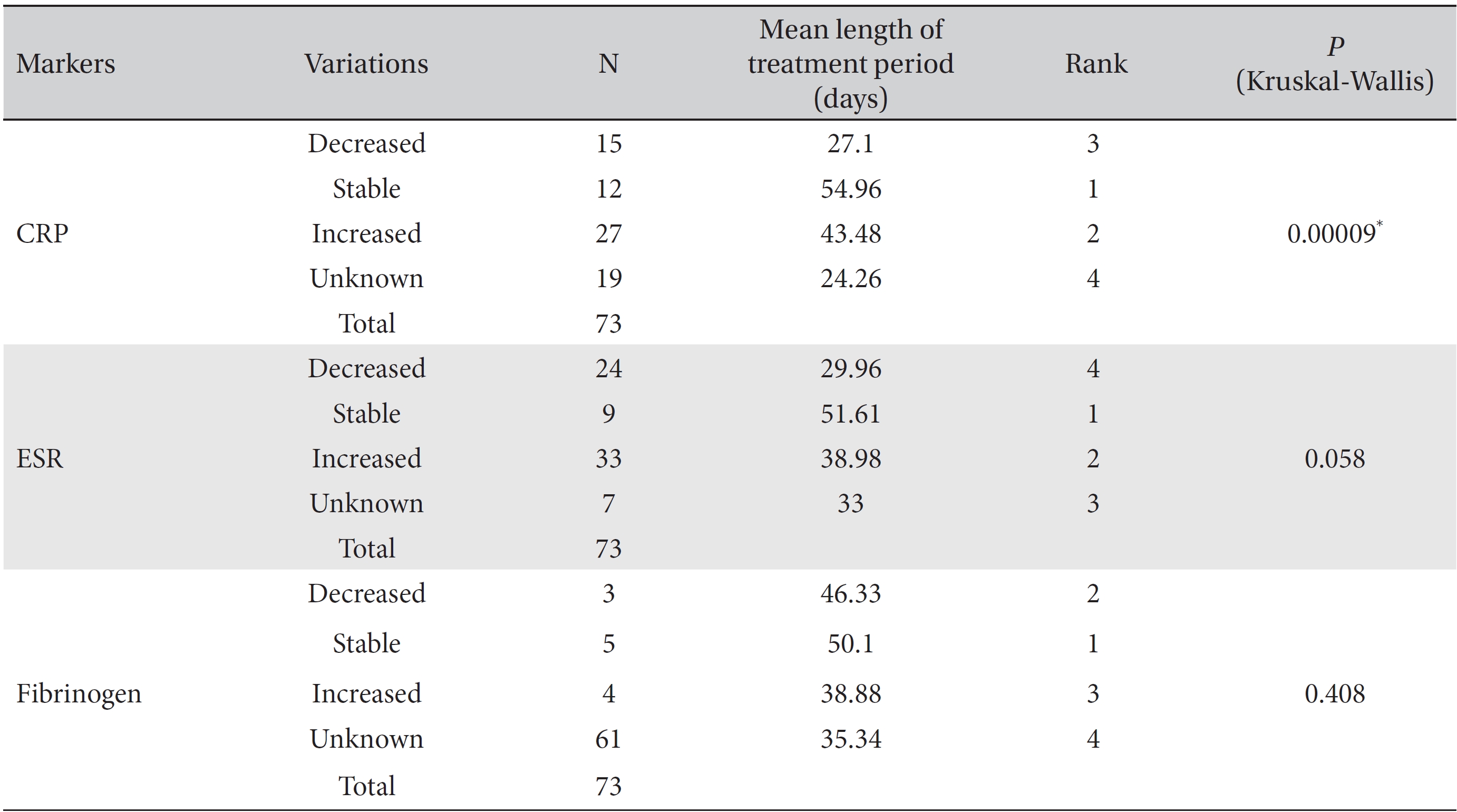
Differences among the treatment period lengths for decreased, increased, stable, and unknown marker levels were analyzed by using the Kruskal-Wallis test and showed statistical significance. The levels of the CRP and the ESR, but not those of fibrinogen showed a statistically significant correlation with the treatment period. In the analysis of the CRP and the ESR, the mean treatment period of the stable level group was found to be the longest, followed by the increased and the decreased level groups (CRP,
3.2. Cross-analyses of the correlations between markers and the treatment period
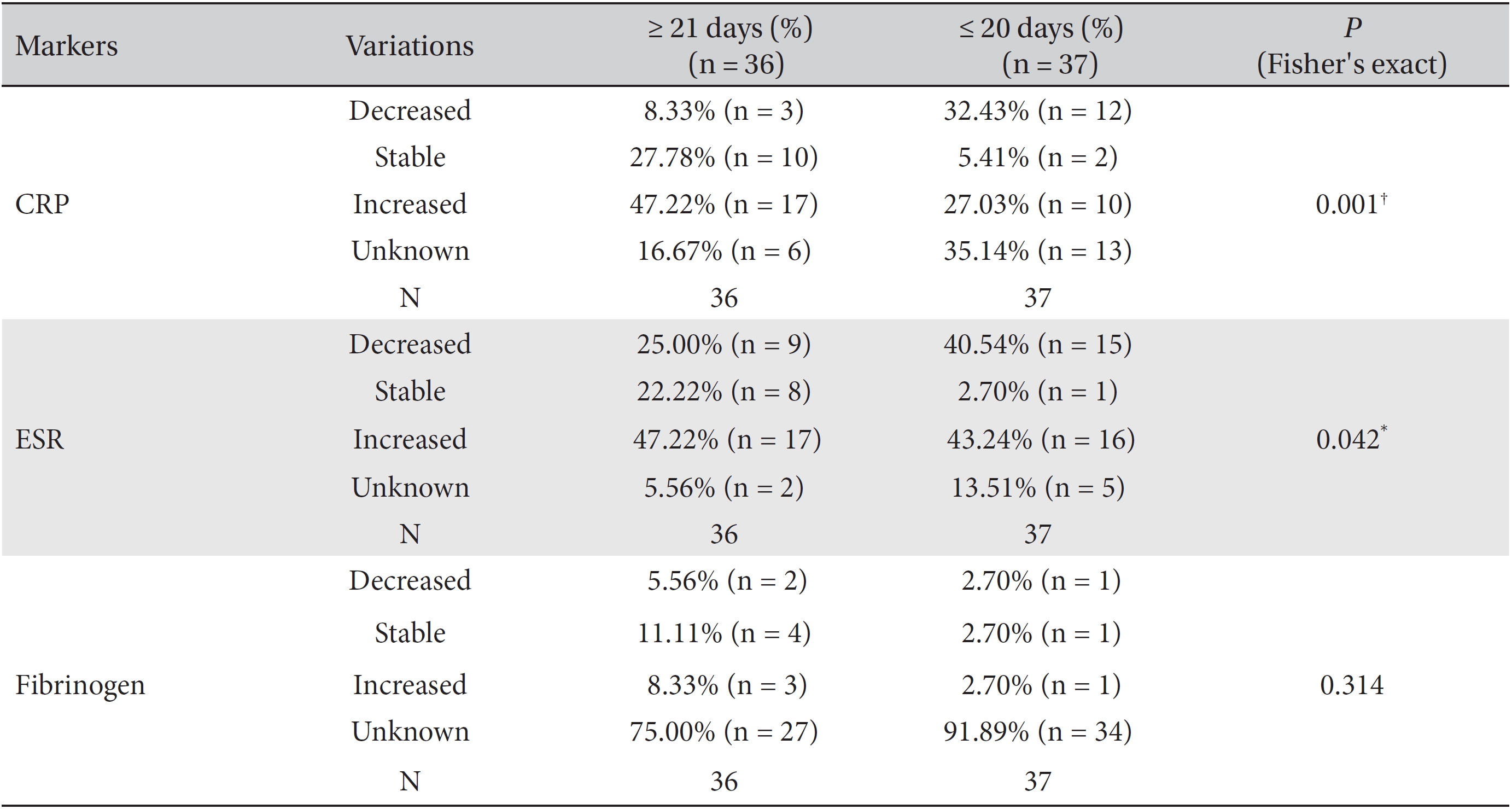
We divided the patients according to the treatment period into the ≥ 21 day group and ≤ 20 day group and analyzed the variations of the markers by using Fisher's exact test and the likelihood ratio test. Differences among the percentile population sizes were analyzed for both the ≥ 21 day group and the ≤ 20 day group and were classified as decreased, increased, stable or unknown. In the case of fibrinogen, the study population was too small for this analysis to make a determination, but statistically significant differences between the two groups were found for CRP and ESR.
More patients with stable or increased CRP levels, but fewer patients with decreased CRP levels, were found in the ≥ 21 day group than in the ≤ 20 day group (Fisher's exact test:
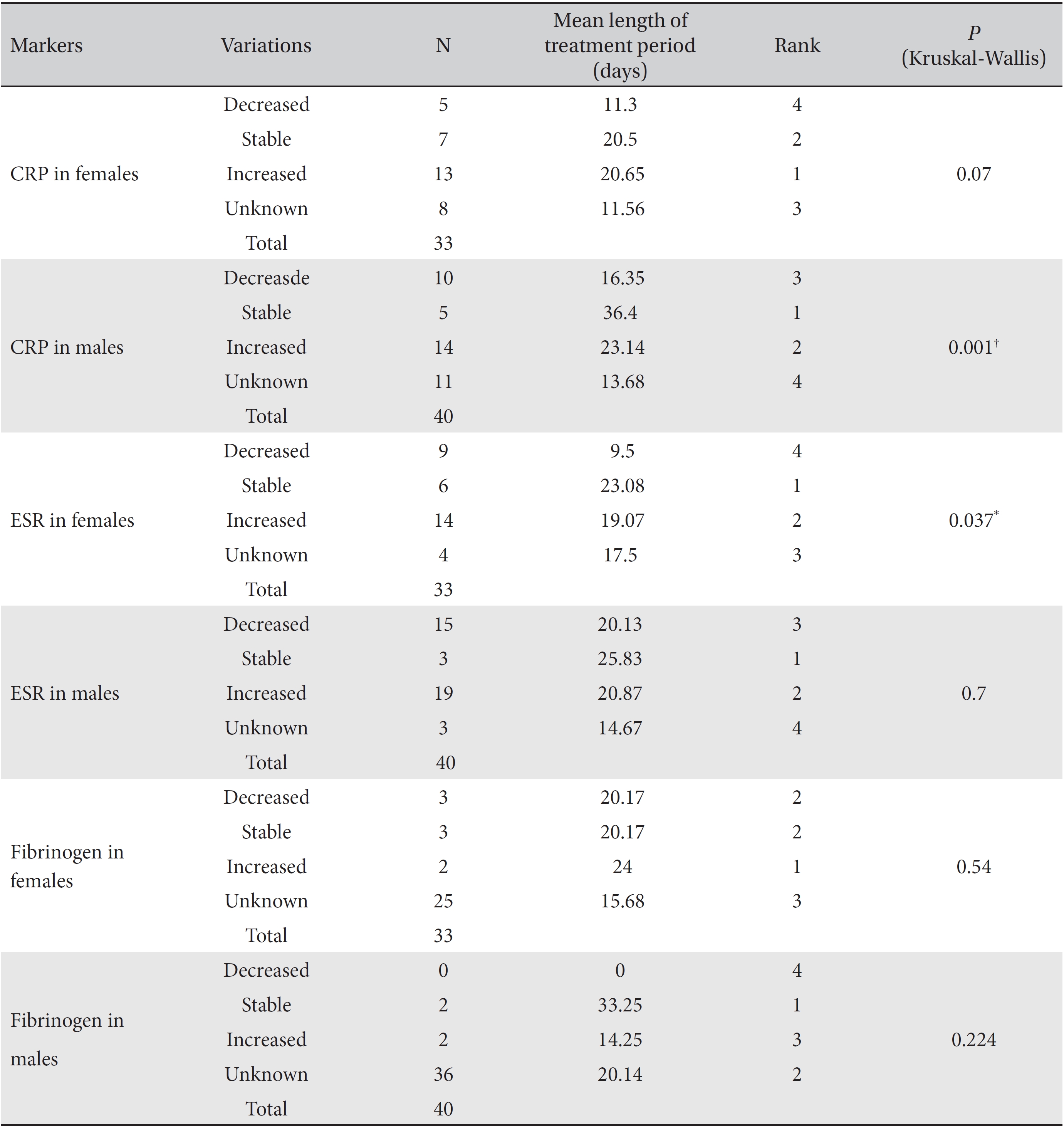
Differences in the markers for different lengths of the treatment period were analyzed by using the Kruskal-Wallis test, and the results were classified as decreased, increased, stable or unknown. In the analysis of the correlation between fibrinogen levels and the length of the treatment period, both female and male subgroups showed no significant differences. In the analysis of CRP, both the female and the male subgroups showed significant differences that depended on the length of the treatment period (females:
In the male group, the mean treatment period necessary to obtain stable CRP levels was longest, followed by the treatment periods for increased and decreased levels. In the female group, the mean treatment period to obtain stable ESR levels was the longest, followed by of the treatment periods for increased and decreased levels. Except for unknown levels, decreased CRP and ESR levels were associated with the lowest mean lengths of the treatment period.
3.4. Cross-analyses of the correlations between markers and the treatment period according to gender
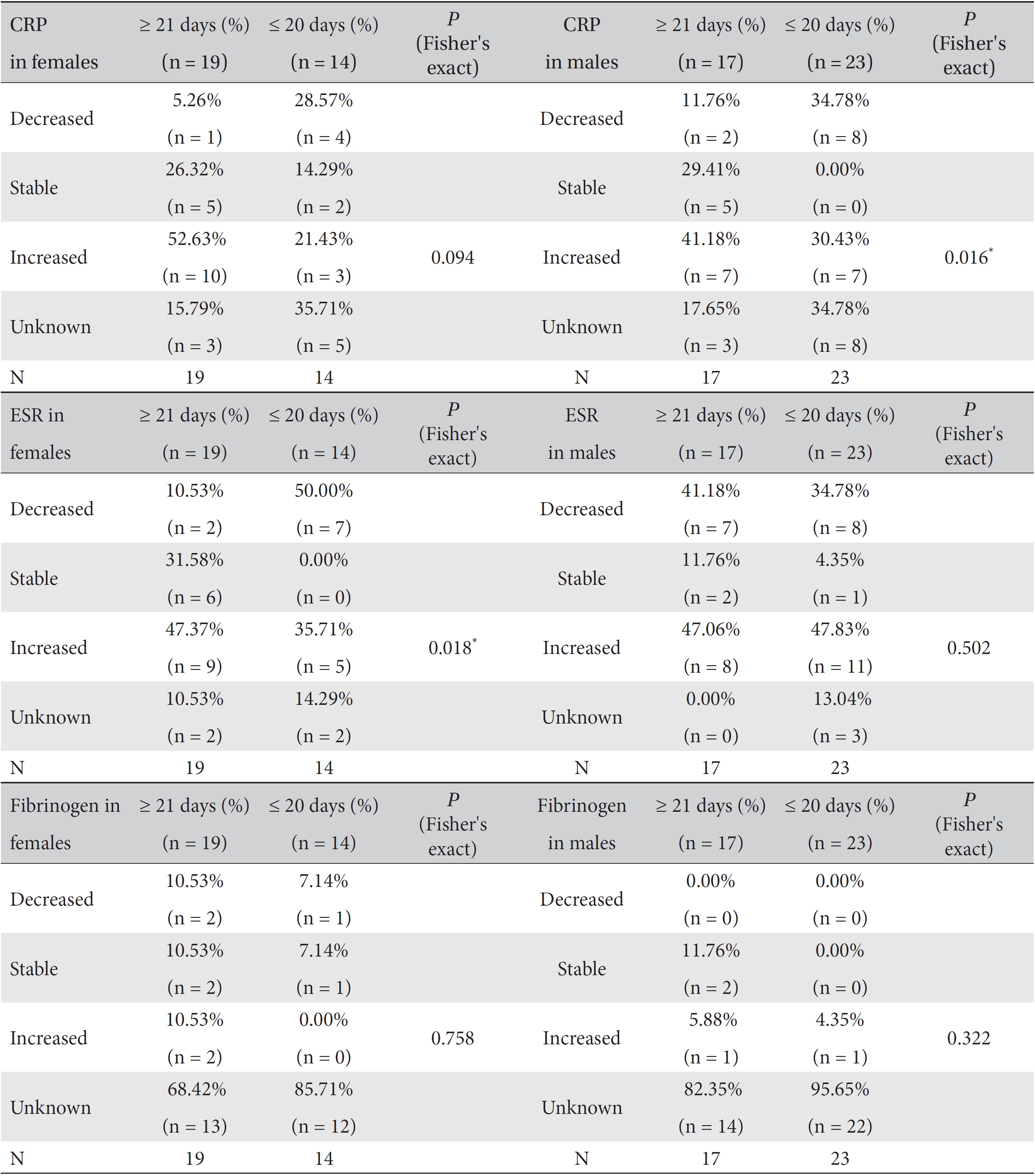
We analyzed the variations in the marker levels according to gender separately in the ≥ 21 day and the ≤ 20 day groups by using Fisher's exact test and the likelihood ratio test. Difference among the percentile population sizes were analyzed in both the ≥ 21 day and the ≤ 20 day groups, and the results were classified as decreased, increased, stable or unknown. ESR in the female subgroup and CRP in the male subgroup showed significant differences between the ≥ 21 day and the ≤ 20 day groups (For ESR in females, Fisher's exact test:
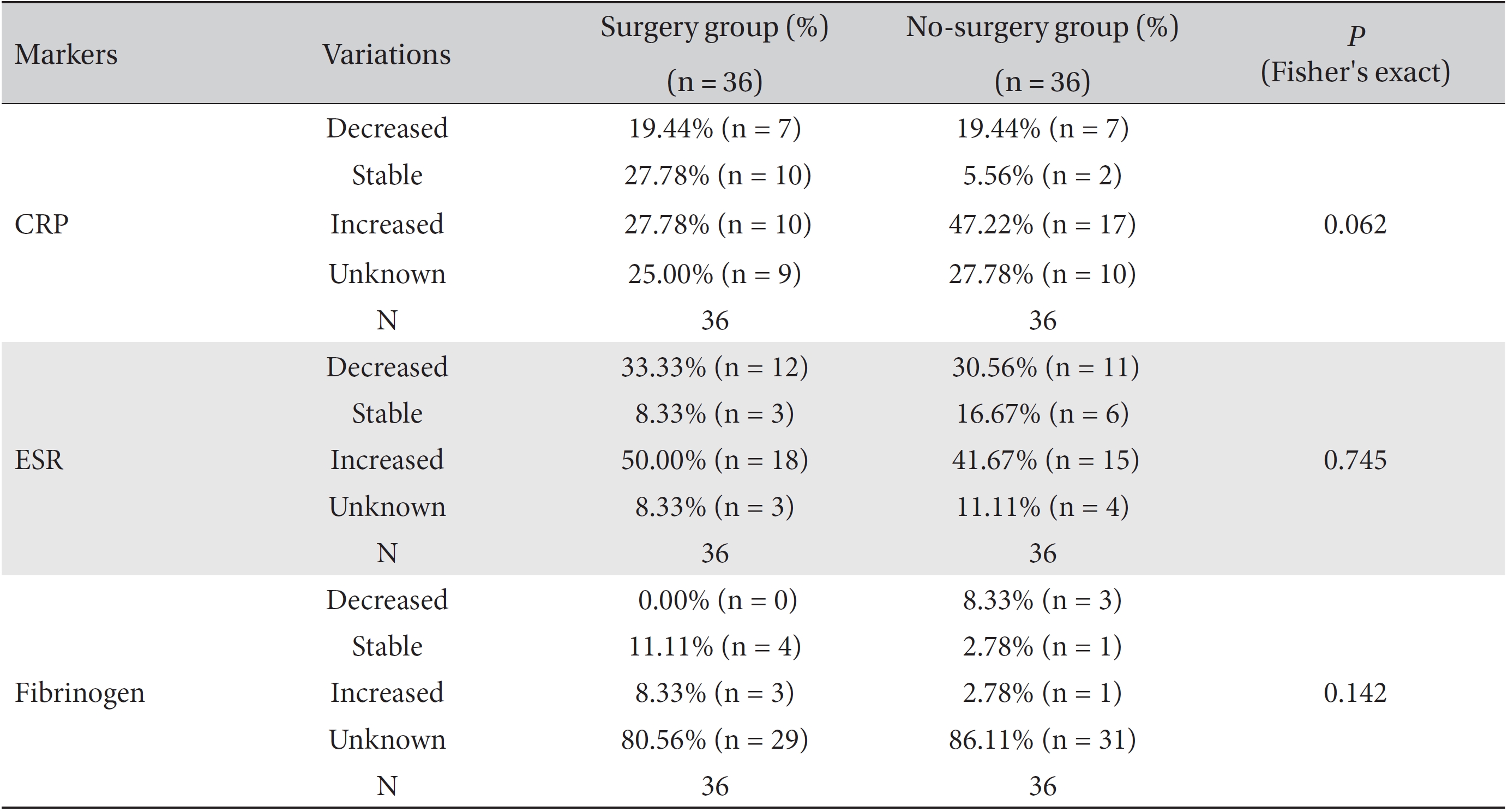
Differences in the marker levels were analyzed for both the group that underwent surgery and the group that did not, and the results were classified as decreased, increased, stable or unknown. No significant differences were observed between these two groups. However, more patients had stable CRP levels, but fewer patients had increased CRP levels, in the surgery group than in the no-surgery group. Both groups had the same proportion of patients with decreased CRP levels (Table 8).
We analyzed the variations in the marker levels separately for the surgery and the no-surgery subgroups in the ≥ 21 day and the ≤ 20 day groups by using Fisher's exact test and the likelihood ratio test, and the results were classified as decreased, increased, stable or unknown. For fibrinogen, neither the surgery or no-surgery subgroup showed significant differences between the ≥ 21 day and the ≤ 20 day groups. For ESR, only the no-surgery subgroup showed a statistically significant difference (Fisher's exact test:
A study on 50 advanced cancer patients found that the CRP levels were significantly elevated in most of them [15]. A study on 106 inoperable NSCLC patients found that the CRP levels were elevated compared to normal controls in almost 80% of the patients [16]. The elevation of the CRP levels in a study on pancreatic cancer patients was significantly greater during 8 weeks before death than during 8 weeks after diagnosis, and the median elevation of the CRP level of all patients was 15 mg/L [17]. Elevated CRP was also significantly associated with advanced International Federation of Gynecologists and Obstetricians (FIGO) stages, with the presence of a postoperative residual mass in 623 epithelial ovarian cancer patients [18], and with the volume of ascites in 120 patients with other forms of ovarian cancer [19]. In a study of 115 gastric cancer patients who underwent a gastrectomy, elevated CRP was significantly associated with invasion depth, advanced tumor node metastasis (TNM) stage, and lymph node metastasis [1]. An elevated CRP level could be used as a poor prognostic factor for survival of stage 0 to IIIA breast cancer patients, preoperative esophageal cancer patients, postoperative gastro-esophageal cancer patients, and hepatocellular carcinoma patients who underwent a curative resection [20-23]. On univariate analysis, an abnormally elevated CRP level was significantly associated with survival whereas on multivariate analysis, it was an independent prognostic factor for cancer-specific survival in colorectal, gastric, breast, and bronchogenic carcinoma patients [24]. Elevated CRP level was also recognized as an independent prognostic factor for NSCLC patients and showed a significant correlation with reduced survival [16].
Fibrinogen is known to be an important factor in metastasis [10]. Cancer was more commonly found in patients with idiopathic deep vein thrombosis than in healthy subjects [25]. This result suggests a correlation between tumor generation and extravasated blood. In addition, fibrinogen impedes NK cell mediated elimination of tumor cells by adhering to NK cells [26]. In a study on 341 colorectal cancer patients who underwent a curative resection, elevated fibrinogen was significantly associated with postoperative distant metastasis, venous invasion, and tumor size [27]. An elevated fibrinogen level was a poor prognostic factor for survival in colorectal cancer patients, endometrial cancer patients, and epithelial ovarian cancer patients [27-29]
Systemic breast cancer patients showed a significant ESR increase in comparison with non-systemic breast cancer patients [30]. In a multicenter clinical trial for early stage Hodgkin disease, an unexplained elevation of ESR after radiotherapy or chemotherapy was suggested as a prognostic factor for early relapse and poor prognosis [31]. In a study on 295 patients with a metastatic renal cell carcinoma treated with chemotherapy or interferon, lower ESR was related to improved survival as an independent prognostic factor [32]. ESR showed at least threefold risk ratios for death in renal cell carcinoma patients in the uniand the multivariate analyses [6]. An abnormally elevated ESR level was an independent prognostic factor for poor survival rates in prostatic cancer and NSCLC patients [7, 8, 33]. In a study on newly diagnosed myeloma patients and normal controls, higher ESR was correlated with poor survival as an independent prognostic factor [34].
As mentioned above, inflammatory and coagulation markers like CRP, ESR, and fibrinogen commonly have abnormally elevated levels in advanced cancer patients, and this effect is more pronounced in patients with terminal phase cancer. In addition, these three markers are associated with poor survival rates of advanced cancer patients as independent prognostic factors. In this study on stage IV cancer patients, the mean WBCT treatment period of the stable level was found to be the longest in the CRP and the ESR levels, followed by those of the increased and the decreased levels in almost all analyses. Considering the tendency of the CRP and the ESR levels to be increased in stage IV cancer patients, this means that long-term WBCT treatment prevents an abnormal elevation of the inflammatory markers CRP and ESR and, furthermore, contributes to the improvement in the patients survival rates. WBCT is a multi modality cancer care program, which is based on the theory of Korean medicine and is provided to EWCC inpatients [35]. In one-way analyses of CRP and ESR, the result for CRP was significant, but that for ESR was not. This might be explained by the different lag times for CRP and ESR to increase in the presence of inflammation [36].
In the analyses of the female and the male groups, only ESR in the female group and only CRP in the male group showed statistically significant results. The mean treatment period of the stable level subgroup was found to be the longest, followed by those of the increased and the decreased level subgroups. We cannot explain why only the ESR level in the female group and only the CRP level in the male group showed significant differences, but these results are presumably related to the different sizes of the populations tested for CRP and ESR in both the male and the female groups. However, the decreased levels of CRP and ESR were usually associated with a shorter treatment period and, thus, are presumably related to a relatively milder illness, which might shorten the necessary treatment period.
In cross analyses of the correlations between the CRP and the ESR levels and the treatment period, the ≥ 21 day group had a significantly higher proportion of patients with stable levels of the markers than the ≤ 20 day group. These results suggest that providing WBCT treatment for longer than 21 days helps to maintain the CRP and the ESR levels and affects patient survival. The one-way analysis of ESR level and the cross analyses of the female CRP level and the CRP levels in the surgery and the no-surgery groups showed
As mentioned above, in almost all cross-analyses of CRP and ESR, the ≥ 21 day group had a higher proportion of patients with increased marker levels whereas the ≤ 20 day group had a higher proportion of patients with decreased marker levels. These results can be interpreted as a bias generated by the relative seriousness of the illness of advanced cancer patients. More specifically, the hospitalization period becomes longer with increasing seriousness of the illness; consequently, the treatment period can become longer, too. In addition, the magnitude of the elevated CRP and ESR levels in patients who have a serious illness tends to be greater than those in patients who have a less serious illness [17, 30].
In the cross analyses of the ESR and the CRP levels in the female and the male groups, the ≥ 21 day group had a high proportion of patients with stable marker levels whereas no such patients existed in the ≤ 20 day group. This result is presumably related to the different sizes of the populations tested for CRP and ESR in the male and the female groups.
In the cross analyses of CRP according to surgery, the surgery group had a higher proportion of patients with stable CRP levels than the no-surgery group. This result can be interpreted as indicating that surgery helped to maintain the CRP level, but it can also be assumed that the surgery group had a less serious illness than the inoperable group; Consequently, the magnitude of the increase in the CRP level was smaller in the surgery group. Similarly, in the cross-analyses of the ESR level in the no-surgery group and the CRP level in the surgery group, the ≥ 21 day group had a higher proportion of patients with stable marker levels than the ≤ 20 day group.
Judging from these results, long-term administration of a herbal decoction helps to maintain the CRP and the ESR levels and, therefore, can have a favorable effect on the prognosis of stage IV patients. Providing WBCT treatment for 21 days or more is more efficient in maintaining the CRP and the ESR levels than providing it for 20 days or less. These results may be associated with the concentration of NK-cells, which is not described in this study and which is presumably related to the pro-coagulation mechanism explained by up-regulation of pro-angiogenic vascular endothelial growth factor and down-regulation of the anti- angiogenic factor thrombospondin triggered by the tissue factor [14].
This study had some limitations. First, all analyses on fibrinogen, an important factor in tumor metastasis and tumor survival, failed because of the small population size. Thus, we look forward to further studies, with larger population sizes, on the association of fibrinogen with the prognosis of advanced cancer patients. Second, a bias was generated by the relative seriousness of the illness of advanced cancer patients. Thus, a need exists to conduct additional studies that separate patients into a few groups by the degree of the advanced or the metastatic state of the cancer. Third, this study did not analyze each primary site of cancer because of the small population sizes when the patients were separated according to the primary site of the cancer. Therefore, additional studies with large population sizes are needed. Fourth, this study was retrospective; thus, we could not evaluate the effects of WBCT treatment as precisely as we could have in a prospective study. Thus, more rigorously designed prospective studies are needed in the future.
We expect that consistently providing WBCT treatment for more than 21 days will have a certain effect on maintaining the CRP and the ESR levels in stage IV cancer patients hospitalized at hospitals that offer traditional Korean medicine. Furthermore, a need exist to prescribe additional herbal decoctions at the time of the patients discharge from the hospital if the hospitalization period of the patient was less than 21 days.
For stage IV cancer patients hospitalized at Korean medicine hospitals, more than 21 days of long-term of WBCT should help maintain stable CRP and ESR levels and should favorably affect survival rates.
[Table. 1] Clinical characteristics of stage IV cancer patients
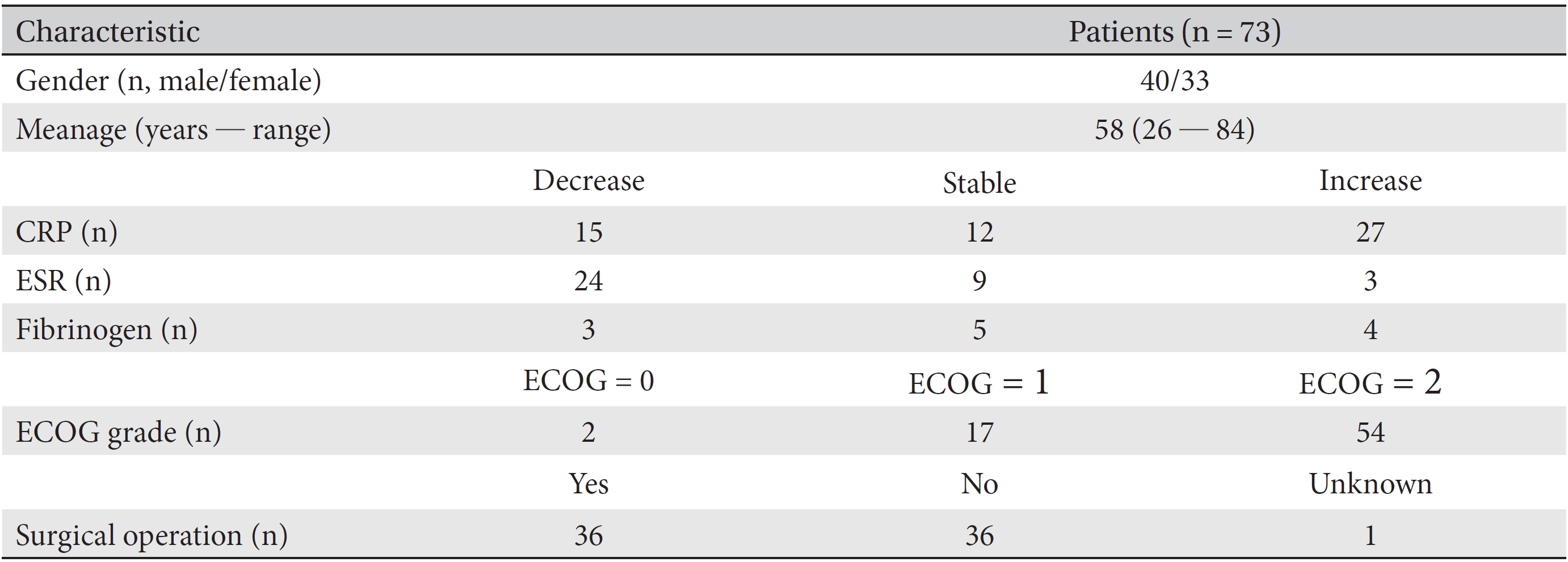
Clinical characteristics of stage IV cancer patients
[Table. 2] Clinical characteristics of patients according to the treatment period and their gender

Clinical characteristics of patients according to the treatment period and their gender
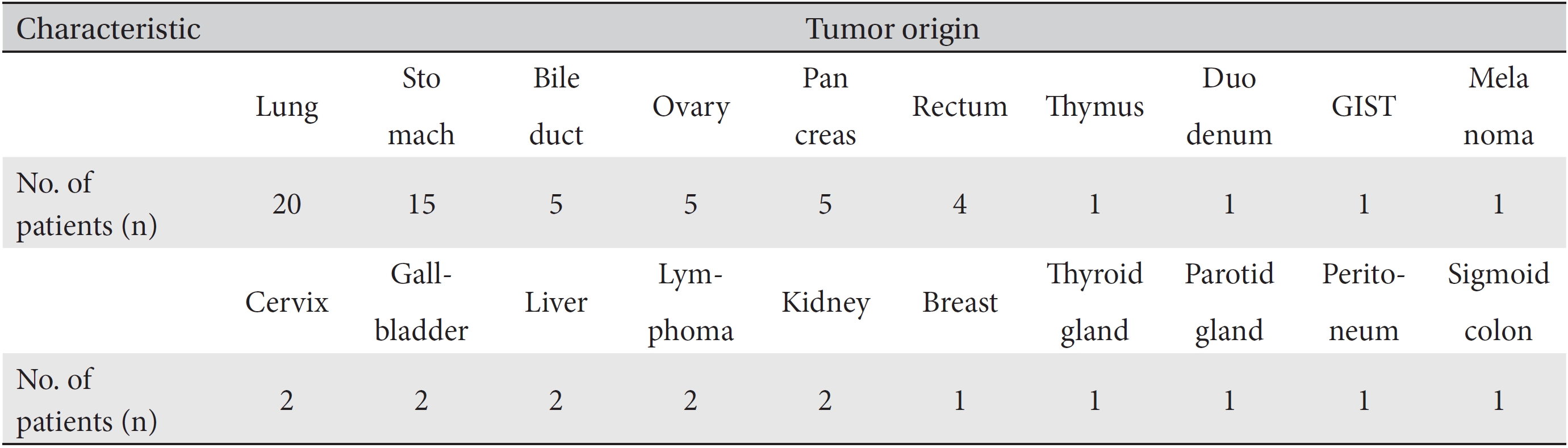
[Table. 3] Tumor origins of patients
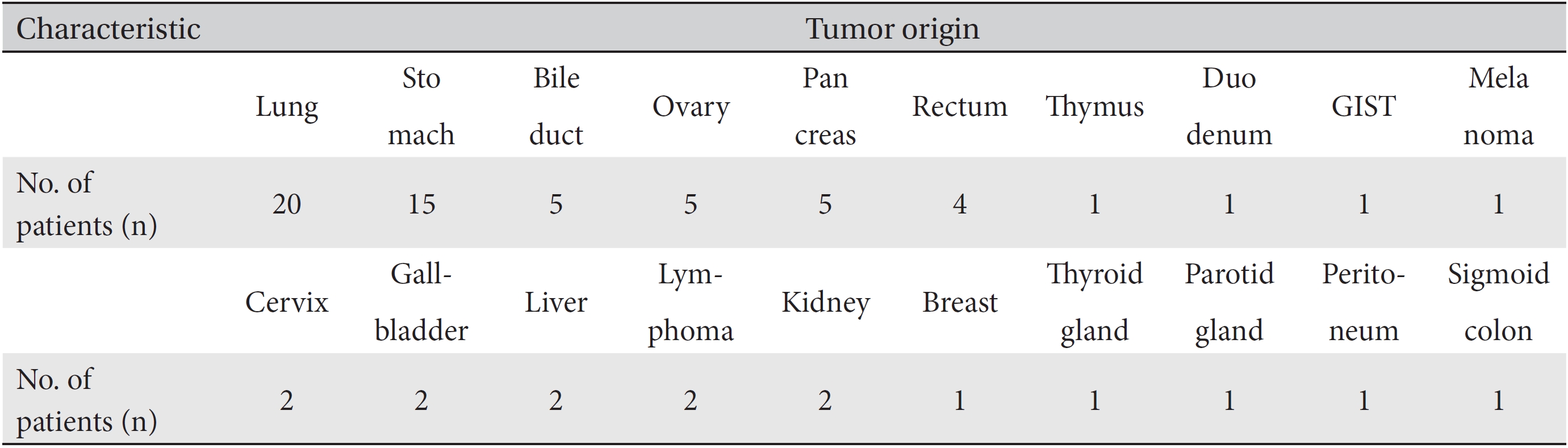
Tumor origins of patients
[Table. 4] Mean treatment periods of the CRP, ESR and fibrinogen levels
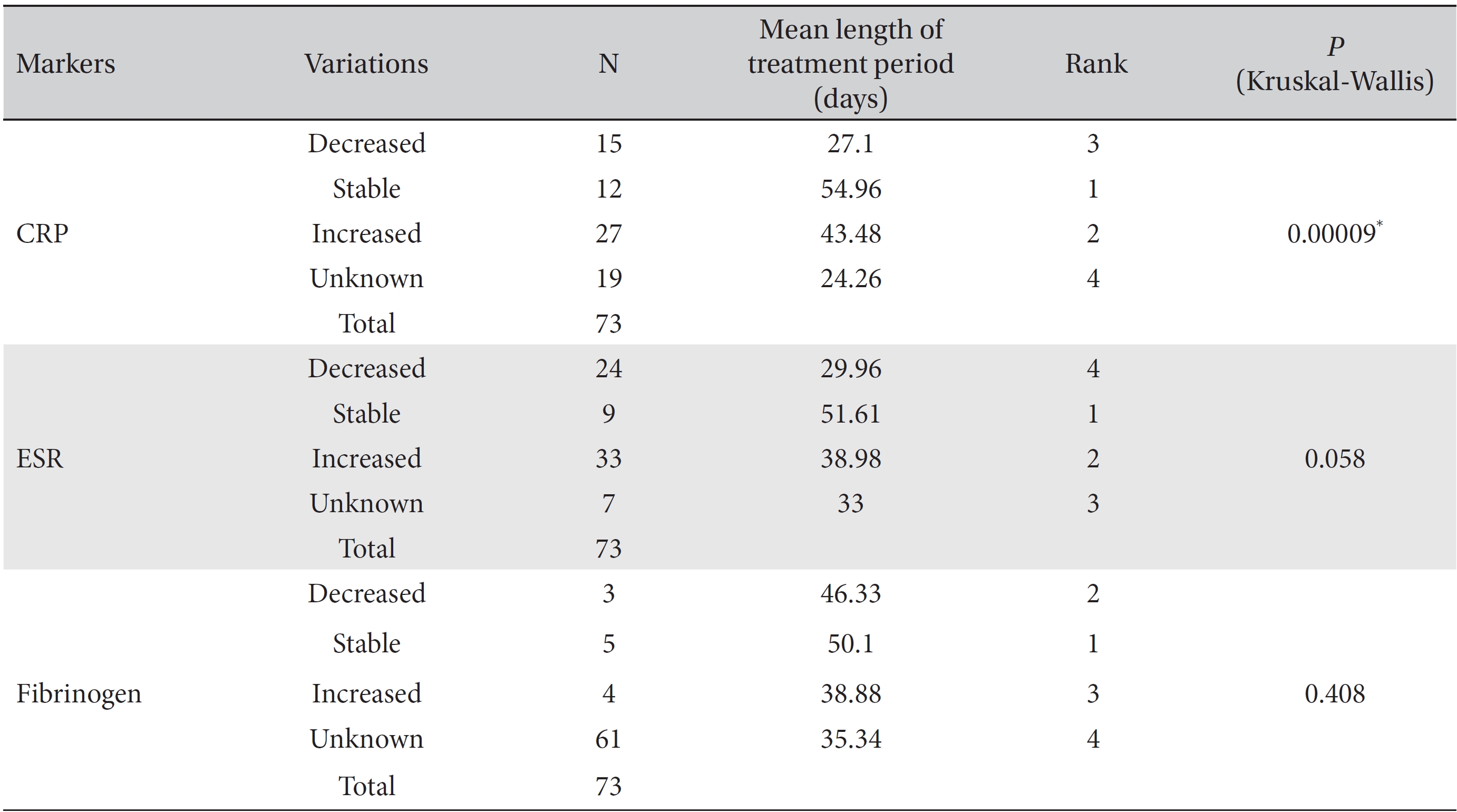
Mean treatment periods of the CRP, ESR and fibrinogen levels
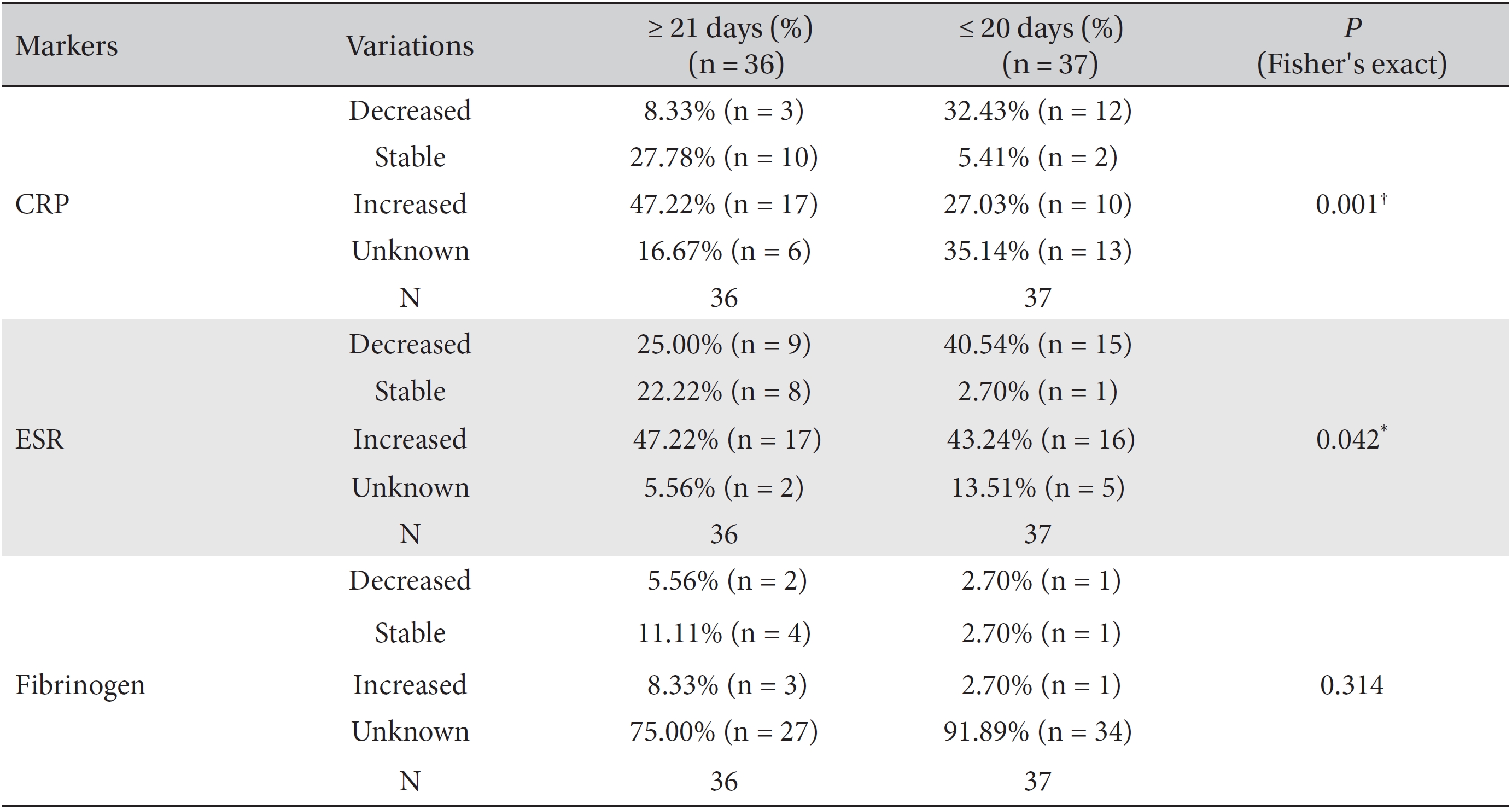
Results of cross-analyses of the CRP, ESR and fibrinogen levels in the ≤ 20 day group and ≥ 21 day group
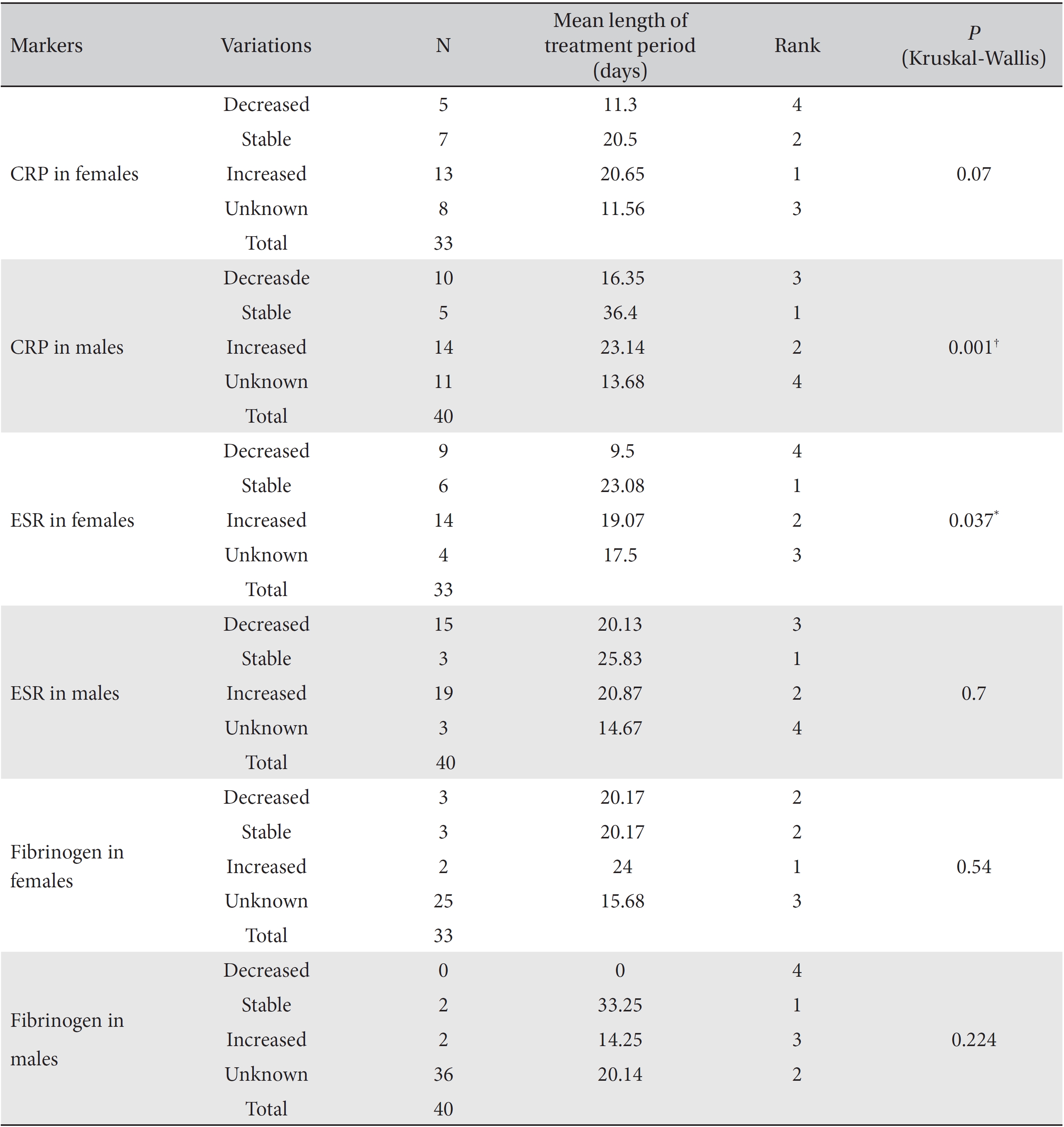
CRP, ESR and fibrinogen levels according to the length of the treatment period in both the female and the male subgroups

Results of cross-analyses of the ESR, CRP and fibrinogen levels in the ≥ 21 day and ≤ 20 day group for both female and male subgroups
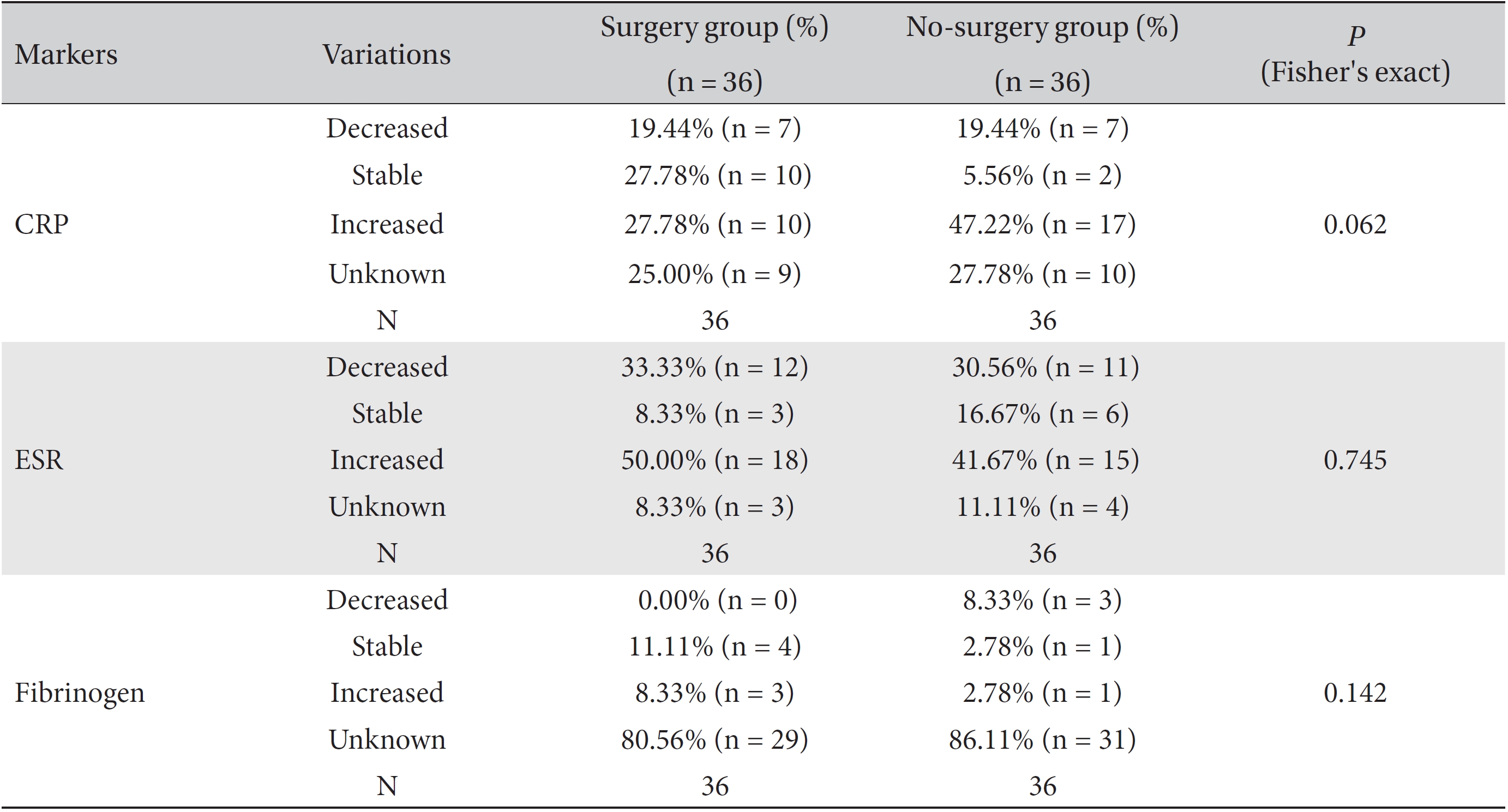
Results of cross-analyses of the ESR, CRP and fibrinogen levels in the surgery and no-surgery groups

Results of cross-analyses of the CRP, ESR and fibrinogen levels in the ≥ 21 day and ≤ 20 day groups for both no-surgery and the surgery subgroups