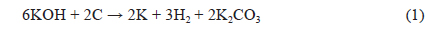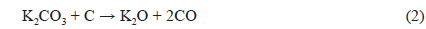Hydrogen has many advantages as an alternative to fossil fuels, as it produces no pollutants, has high energy efficiency, and is essentially an unlimited resource [1-3]. However, its storage presents a problem. Many hydrogen storage methods have been extensively investigated, including metal hybrids, liquid hydrogen, high-pressure hydrogen, and adsorption [4-7]. Among these storage methods, adsorption by carbon materials has many advantages such as good heat resistance, chemical stability, reversibility, and low cost. Many carbon materials for adsorption have also been reported, and include activated carbon, carbon aerogels, graphene, and carbon nanotubes [8-12].
Hydrogen storage capacity is strongly influenced by the specific surface area and microporosity of the adsorbents [13]. Therefore, various methods for the synthesis and surface modification of porous carbons have been developed; for example, templating with other porous materials such as zeolites, metal-organic frameworks (MOFs), and silica [14-17], and direct carbonization of various precursors. Template methods are complicated because they involve the removal of the templated materials. Direct carbonization uses environmentally friendly precursors from agricultural wastes such as rice husks, corncobs, and coconut shells [18,19]. The use of these precursors is also helpful in preventing pollution caused by agricultural wastes. However, biomass-derived carbons (BCAs) have a relatively low specific surface area, and therefore they require activation to increase their specific surface area and micropore volume.
There are two activation methods that are used for BCAs: 1) chemical activation and 2) physical activation [20,21]. Chemical activation is conducted using acidic or basic chemicals such as H3PO4, KOH, Na2CO3, and NaOH and can lead to high specific surface area and microporosity as a consequence of erosion and oxidation. Physical activation requires an oxidizing gas, such as water vapor, carbon dioxide etc.
In this study, we prepared biomass-derived aerogels from watermelon flesh using a hydrothermal reaction, followed by carbonization. Chemical activation was conducted at various temperatures using KOH to enhance the hydrogen storage capacity of the aerogels.
2.1. Materials and preparation
Biomass-derived aerogels were prepared by the hydrothermal reaction of watermelon flesh; the details have been provided elsewhere [22]. The watermelon flesh was cut and put into a 100-mL Teflon-lined autoclave. The hydrothermal reaction was conducted at 180℃ for 12 h, and then the sample was immersed in a mixture of distilled water and ethanol for several days to remove the impurities. After drying at 80℃ for 12 h, the obtained biomass-derived aerogels were carbonized under flowing N2 (200 mL/min) at 550℃ for 1.5 h. The resulting BCA was mixed with KOH (mass ratio of 2), heated to a target temperature in the range 700℃-900℃ in a tubular furnace under N2 atmosphere (200 mL/min), and maintained at the target temperature for 1 h. After cooling down to room temperature, the resulting materials were taken out and washed with distilled water and hydrochloric acid until the pH became neutral. The KOH-activated BCA was labeled K-T-BCA, where T denotes the activation temperature.
X-ray diffraction (XRD) measurements were carried out on a Bruker-AXS D2 Phaser Desktop X-ray Diffractometer with a Lynx-Eye detector using CuKα radiation at 30 kV and 10 mA (λ = 1.5406 Å). The patterns were measured with a scan step time of 3 s and step size of 0.02°. The scanning electron microscope (SEM) images were obtained using Model S-4300SE (Hitachi Co., Ltd.). The textural properties were investigated by nitrogen adsorption-desorption isotherms at -196℃ using a gas adsorption analyzer (Model BEL-SORP, BEL Co., Ltd.). The samples were degassed at 200℃ for 6 h before the measurement. The hydrogen storage capacity was measured by using a gas adsorption analyzer (Model BEL-SORP, BEL Co., Ltd.) at 77 K and 1 bar. We used ultrahigh purity hydrogen (99.9999%) in order to reduce the influence of other impurities and used the volumetric measurement method to determine the hydrogen storage capacity.
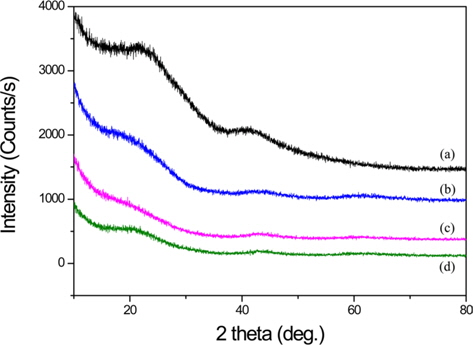
The XRD patterns of BCA and K-T-BCAs are shown in Fig. 1. They show typical and almost identical carbon peaks. After chemical activation, the peak intensities for carbon at 23° and 44° decreased, because KOH destroys the microstructure of the biomass-derived activated carbons. This mechanism of activation by KOH has been reported previously [23,24] and is summarized below:
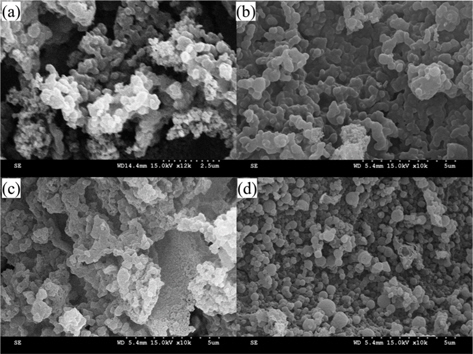
Potassium is produced during K2O activation at a high temperature, and it was found that the formation of pores was due to the loss of carbon. The SEM images that are shown in Fig. 2 also indicate the effects of the activation temperature. It can be seen that KOH activation changed the sample particle size and fineness. K-900-BCA has fine particles and regular granules compared with the other K-T-BCAs and BCA. This might be attributed to the erosion of BCAs caused by the higher activation temperature.
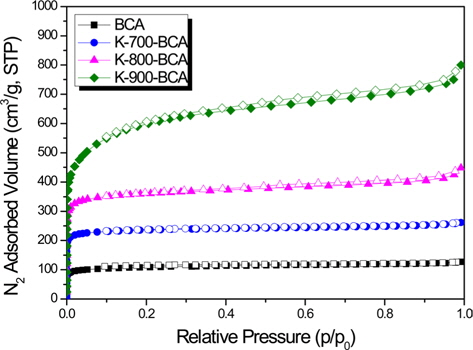
Fig. 3 shows the nitrogen adsorption-desorption isotherms at 77 K. BCA and K-T-BCAs appear to have Type 1 isotherms without a noticeable hysteresis loop, which is typical of physisorption in microporous materials [25]. Most of the pores were filled below a relative pressure of about 0.1, indicating that these samples have high microporosity. However, K-800-BCA and K-900-BCA show a slight hysteresis loop and increased adsorption volume of nitrogen at a relative pressure of about 1.0. These results show that the microstructure was destroyed and the macropores were formed at higher activation temperatures.
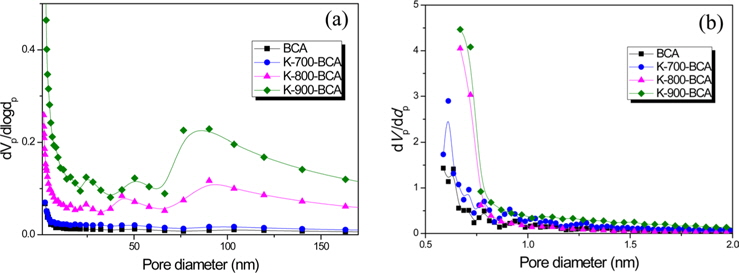
Fig. 4 shows mesopore and micropore size distributions of BCA and K-T-BCAs. The BCA and K-T-BCAs have similar peaks, but K-900-BCA has the highest peak intensity with most pores <1 nm. Moreover, K-900-BCA shows a relatively high peak intensity above a pore diameter of 50 nm; these results correspond with Fig. 3.
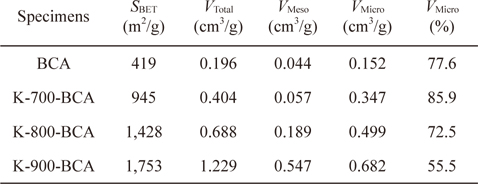
In order to understand the pore structures of the BCA and K-T-BCAs in detail, Table 1 shows the textural properties of BCA and K-T-BCAs. The specific surface area, total pore volume, and micropore volume of K-T-BCAs were influenced by the activation temperature. The specific surface area and pore volume of the samples increase as the activation temperature was increased. As expected, K-900-BCA has the highest specific surface area (1,753 m2g-1), total pore volume (1.229 cm3g-1), and micropore volume fraction (72.5%). The high specific surface area and good micropore volume fraction provide good conditions for hydrogen adsorption.
[Table 1.] N2/77 K textural properties of BCA and K-T-BCAs
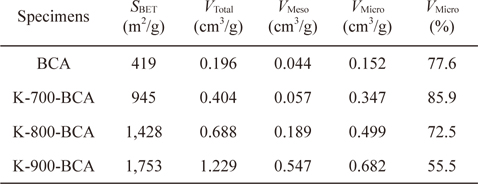
N2/77 K textural properties of BCA and K-T-BCAs
3.2. Hydrogen storage capacities
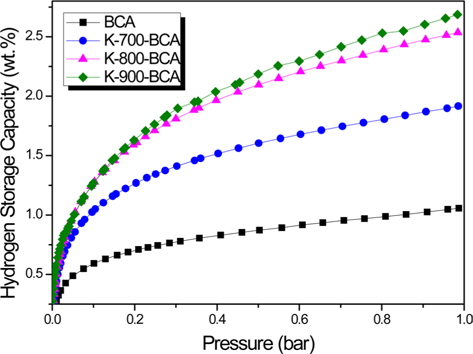
Fig. 5 shows the hydrogen storage capacities of BCA and K-T-BCAs at 77 K and 1 bar with various sample activation temperatures. K-900-BCA has the highest hydrogen storage capacity of 2.7 wt%. The corresponding values for K-800-BCA, K-700-BCA, and BCA are 2.5, 1.9, and 1.1 wt%, respectively. As can be seen from the results of textural properties, K-900-BCA has the highest specific surface area and micropore volume, and therefore, it has the highest hydrogen storage capacity. These results show that KOH greatly affects carbons at high temperatures. The hydrogen adsorption of the porous carbon materials strongly depended on their microporosity, and the results of these experiments correspond with an earlier report [26]. The hydrogen storage of BCA and K-T-BCAs was also influenced by the microporosity of the samples, which can be enhanced by activation.
In this study, we prepared biomass-derived activated carbons using watermelon flesh and a hydrothermal reaction, followed by chemical activation with KOH at various temperatures. The obtained porous carbons had specific surface areas in the range 419-1753 m2g-1 and micropore volumes in the range 0.044-0.547 cm3g-1. The results show that the specific surface area and micropore volume were influenced by the activation temperature. K-900-BCA showed the highest specific surface area (1753 m2g-1) and micropore volume (0.547 cm3g-1). Therefore, K-900-BCA has the highest hydrogen storage capacity, amounting to 2.7 wt% at 77 K and 1 bar. Thus, K-900-BCA can be used as a potential hydrogen storage material and can be enhanced with other metal doping or surface functionalization to increase its hydrogen storage capacity.