Carbon dioxide (CO2) emissions into the atmosphere have increased due to the combustion of fossil fuels in the operation of factories and automobiles. CO2 is a greenhouse gas that gives rise to critical environmental issues, including global warming. Reduction of CO2 emissions is thus especially urgent [1,2]. Among many ways to reduce CO2 in the atmosphere, using an absorbent is much simpler than other methods such as CO2 capture and storage technologies involving pre-combustion capture, oxy-fuel combustion, and postcombustion capture [3].
Porous materials, such as activated carbon, zeolites, and molecular sieves, have been used as CO2 absorbents due to their highly developed porous structure [4,5]. In particular, activated carbon has been widely used because of its low cost, hydrophobicity, and flexibility in terms of its porous texture and surface properties [6]. Activated carbon has been prepared from numerous carbonaceous source materials, such as walnut shells, coconut husk, tar pitch, and petroleum pitch. It has also been prepared from renewable resources, such as aluminum industry waste, bottom ash, peat, steel-plant slag, and beer lees [7,8]. In addition, activated carbon may be produced from pitch-based cokes, which may in turn be derived from waste generated during the carbon/carbon composite manufacturing process for highquality brake discs used in F1 automobiles and airplanes. Such cokes have high crystallinity because they are obtained at temperatures lower than 1273 K and extremely high pressure (approximately 700 bar). These cokes can potentially be used as activated carbon and CO2 adsorbents.
In this study, activated carbon was prepared from high crystallinity cokes using various concentrations of potassium hydroxide (KOH) solution. The CO2 adsorption characteristics of coke-based activated carbon were evaluated at different temperatures. Additionally, the crystal, textural, and chemical properties of the coke-based activated carbon were investigated.
The pitch-based cokes used in this study are obtained from a carbon/carbon composites production process at temperature lower than 1273 K under extremely high pressure (approximately 700 bar). The size of the cokes is controlled below 75 μm using a general pulverizer and a sieve. KOH (95.0%, Samchun, Korea) is used to prepare a KOH solution, which is used as an activation agent in this study.
2.2. Preparation of high crystallinity coke based activated carbon
The pulverized cokes are treated with KOH solution to obtain activated carbon. Cokes (2.5 g) are mixed with 18 mL 2, 4, and 8 M KOH solution, respectively. The mixtures of cokes and KOH solution are heated in an electric furnace. Heat treatment is conducted at 1023 K for 3 h under a nitrogen atmosphere. The treated cokes samples are identified as hc-AC-2K, hc-AC-4K, and hc-AC-8K depending on the concentration of KOH solution. The experimental procedure is summarized in Scheme 1.
2.3. Characterization of prepared samples
The crystal structures of the prepared cokes-based activated carbon are investigated using X-ray diffraction (XRD, D8 DISCOVER, Bruker AXS, Germany). The XRD analysis is conducted using a Cu target. The specific surface area and pore structure of the prepared activated carbon are evaluated using N2 adsorption at 77 K with an ASAP 2020 device (Micromeritics, Inc., Corp., USA) after each sample is degassed at 423 K for 3 h. The total pore volume is calculated using a t-plot. X-ray photoelectron spectroscopy (XPS, Thermo Electron Cooperation, MultiLab 2000 spectrometer, England) is performed to investigate the elemental composition of the prepared activated carbon.
The textural properties and pore volume of the prepared cokes-based activated carbon are evaluated using an ASAP 2020 device (Micromeritics Inc., Corp.) using CO2 (3.3 Å) gas because the pore size of activated carbon is extremely small (diameter of N2 gas is 3.8 Å) [3]. The cumulative pore size distribution (PSD) of the prepared activated carbon is calculated using density functional theory (DFT) using DFT plus software supplied by Micromeritics [9]. The CO2 adsorption capacity is measured by CO2 isothermal adsorption at 273 and 298 K at 1 atm.
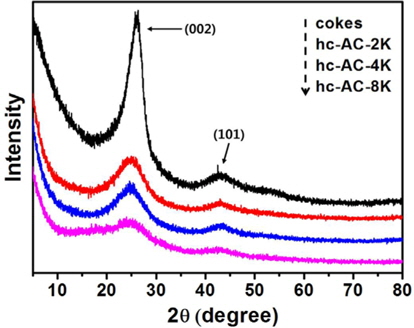
The pitch-based cokes have a sharp (0 0 2) peak at 26° and a (1 0 1) peak at 43°, as shown in Fig. 1. These properties indicate that the pitch-based cokes are high-crystallinity cokes compared to cokes prepared at 1473 K [10], which may be attributed to the high temperature and pressure treatment used for the pitch. After KOH activation, the height of the (0 0 2) peak decreased considerably with an increase of KOH concentration. This decrease indicates that the high K+ (potassium cation) content resulted in attack of the carbon atoms in the crystallite with the (0 0 2) crystal plane, which is the main orientation and then the crystallite with the (0 0 2) orientation decreased [11]. The specific surface area appeared to decrease as the concentration of the KOH solution increased. The height of the (1 0 1) peak changed only slightly. The XRD parameters for La and interplanar spacing were determined to be 0.161 nm and 0.343 nm, respectively, for cokes; 0.095 nm and 0.345 nm, respectively, for hc-AC-2K; 0.114 nm and 0.361 nm, respectively, for hc-AC-4K; and 0.083 nm and 0.37 nm, respectively, for hc-AC-8K.
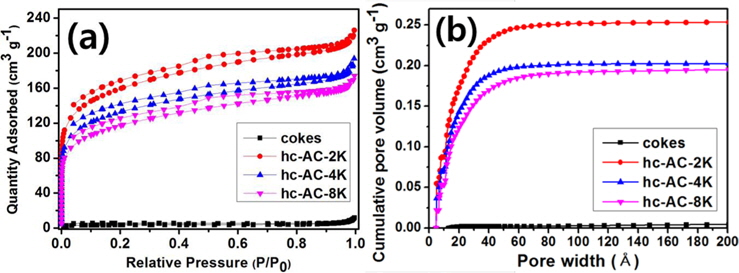
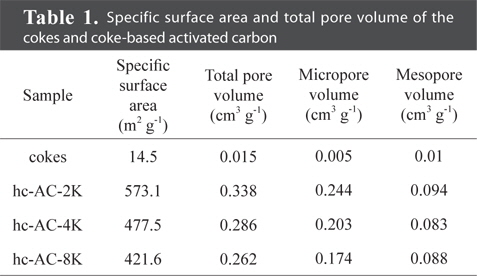
Fig. 2a presents the nitrogen adsorption isotherms of the cokes and the coke-based activated carbon. The isothermal curve of the cokes did not increase as P/P0 increased, indicating non-porous carbon. However, the coke-based activated carbon prepared under different KOH conditions exhibited a significant increase in adsorption, particularly in the region below 0.1 P/P0. The observed isotherms of the coke-based activated carbon were type IV according to the International Union of Pure and Applied Chemistry (IUPAC) classification [12,13]. In addition, the activated cokes exhibited hysteresis, which is H4 type [12]. These isotherm results indicated that the coke-based activated carbon was porous carbon with slitlike pores. These slit-like pores are favorable for adsorbing CO2 gas, given its linear molecular structure. The calculated specific surface areas of the prepared activated carbon are shown in Table 1. The nitrogen adsorption quantity and specific surface area decreased with increased concentration of the KOH solution, which is in accordance with the XRD results presented in Section 3.1.
The cumulative PSDs of the cokes and activated carbon are shown in Fig. 2b. These PSD results indicate that the KOH-treatment of cokes mainly produced micropores whereas untreated cokes had no pores. The main pore size range that affects CO2 adsorption is below 8 Å [14]. In this study, hc-AC-2K was found to have the greatest pore volume within 8 Å were introduced in this study. The pore volume decreased with increased concentration of the KOH solution. This relation indicates that hc-AC-2K has the highest CO2 adsorption capacity in this study. The total pore volume values are also presented in Table 1. The total pore volumes of the cokes, hc-AC-2K, hc-AC-4K, and hc-AC-8K were 0.015, 0.337, 0.285, and 0.261 cm3 g-1, respectively. hc-AC-2K had a higher total pore volume than hc-AC-4K and hc-AC-8K. Considering the cumulative PSD, a high pore volume is attributed to the formation of mesopores, but it is believed that mesopores do not affect CO2 adsorption.
3.3. Surface chemical element composition
To investigate the chemical composition of the activated cokes under different conditions, a XPS analysis was performed to compare the amounts of oxygenated functional groups. Table 2 shows the C1s and O1s elemental content. In the resulting XPS spectra of the activated cokes, the elemental contents of carbon and oxygen atoms were determined to be 94.68% and 5.32%, respectively, for hc-AC-2K; 94.34% and 5.66%, respectively, for hc-AC-4K; and 88.22% and 11.78%, respectively, for hc-AC-8K. The elemental contents of the hc- AC-2K and hc-AC-4K were similar, although the total pore volume of hc-AC-2K was higher than that of hc-AC-4K. In contrast, the oxygen content of hc-AC-8K was considerably higher than that of hc-AC-2K. The oxide group content increased according to the concentration of the KOH solution used, and the corresponding increases in oxide groups are compared in Table 3. Generally, activated porous carbon materials, activated carbon and activated carbon fiber, have several carbon-oxygen conformations after activation with oxidants [15]. The peak at 284.5 eV is assigned to graphitic C (sp2) [16,17]. The values of the cokes and activated cokes in this study are higher than those observed for other activated carbons. Additionally, the contents of the C-O and C=O bonding groups in these samples, indicated by peaks at 286.4 and 287.4 eV, are lower than those in other activated carbons [3]. The amount of C-O and C=O bonds increased with KOH concentration, reaching 6.11% and 5.00%, respectively, for hc-AC-2K; 6.81% and 5.41%, respectively, for hc-AC-4K; and 7.57% and 5.91%, respectively, for hc- AC-8K. The (C=O)OH bonds at 288.8 eV decreased with increased concentration of the KOH solution due to weak bonding. These differences in the surface chemical composition are expected to affect CO2 adsorption on the cokebased activated carbon.
3.4. CO2 adsorption characteristics
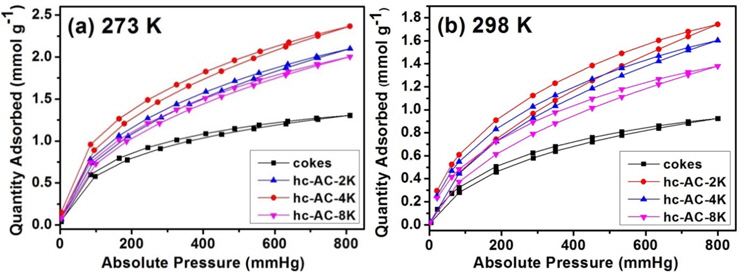
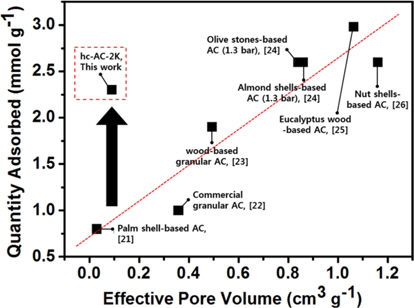
Fig. 3 shows the isothermal CO2 adsorption characterizations of the cokes and coke-based activated carbon at 273 and 298 K, respectively, at pressure ranging from vacuum to 800 mmHg. The quantity of adsorbed CO2 on all samples increased with increasing pressure because physical adsorption depends strongly on pressure. The characterization of porous materials with a substantially microporous size distribution can be expected to increase with increasing pressure [18]. As shown in Fig. 3a, the activated cokes with developed ultramicropores (<7 Å) exhibited the highest adsorption capacity. The pore structure in the high crystallinity coke-based activated carbon is slit-like, similar to the molecular structure of CO2. This slit-like pore structure contributes to higher CO2 adsorption that is comparable to that of activated carbon prepared from various carbonaceous sources, as shown in Fig. 4 [19-24]. In Fig. 4, the results for hc-AC-2K indicate that 1) the high-crystallinity coke-based activated carbon has higher CO2 adsorption than the general CO2 adsorption trend according to pore volume and 2) high-crystallinity cokebased activated carbon provides higher CO2 adsorption than that of nut-shell based-activated carbons, although the former/latter had a higher pore volume of 1.16 cm3 g-1. In addition, the cokes with few pores have a CO2 adsorption capacity below 1 mmol g-1. This phenomenon can be attributed to the ability of the cokes with high crystallinity to interact with CO2 gas. CO2 molecules consist of two oxygen groups with two lone pair groups. These lone pairs can interact with graphitic carbon (sp2) by electrostatic attraction. The amount of CO2 adsorbed on the coke-based activated carbon decreased with increasing temperature due to their weak interaction following increased CO2 activity. However, the CO2 adsorption behavior on hc-AC-2K and hc-AC-4K at 298 K was different from the behavior on coke at 273 K. The curves during initial CO2 adsorption were similar until reaching 200 mmHg, although hc-AC-2K had a larger quantity of ultra-micropores. This difference in the adsorption curves indicates that the CO2 adsorption capability associated with functional groups formed on the activated carbon was greater at higher temperature [3]. The oxygen groups (C=O, C-O, (C=O)OH) introduced onto the carbon surface by KOH activation were electrophilic, which strongly influences the interaction of the material with CO2 [25,26]. This strong relationship arises because oxygen groups are strong electron-donating groups and the carbon atom of CO2 has a positive charge due to the high electronegativity of the oxygen atom. These factors are favorable for electrostatic interaction. The C=O and C-O contents of hc-AC-4K were higher than those of hc-AC-2K. These functional groups increased the initial CO2 adsorption at higher temperature (298 K). Moreover, high-crystallinity cokes can contribute to high CO2 adsorption if they have more developed pore structures and greater pore size.
Pitch based-cokes were activated by using a KOH solution to obtain activated carbon to absorb CO2. The non-activated cokes had a high crystal structure and no pores, as indicated by XRD patterns and nitrogen adsorption isotherms. The coke-based activated carbon had the highest specific surface area and pore volumes within 8 Å when treated with a 2 M KOH solution. Slitshaped pores were formed in the high-crystallinity coke-based activated carbon, which allowed a higher quantity of CO2 to be adsorbed compared to other activated carbons. In addition, the coke-based activated carbon had the highest CO2 adsorption at 273 K. However, at 298 K, the curve of coke activated with a 4 M KOH solution during initial CO2 adsorption was similar to that observed for coke activated with a 2 M KOH solution. This indicates that the oxygen functional groups, i.e., C=O and C-O bonds, are more important than the pore structure at higher temperature.