In this study, carbonized fibers were prepared by using acidically cross-linked LDPE fibers. The surface morphologies of the carbonized fibers were observed by SEM. The effects of cross-linking process temperatures were studied using thermal analyses such as DSC and TGA. The melting and heating enthalpy of the fibers decreased as the cross-linking temperature increased. The cross-linked fibers had a carbonization yield of over 50%. From SEM results the highest yield of carbonized LDPE-based fibers was obtained by cross-linking at a sulfate temperature (170℃). As a result, carbonation yield of the carbonized fibers was found to depend on the functions of the cross-linking ratio of the LDPE precursors.
Carbon fiber is a superb material for applications in the aerospace, automobile, and sporting goods industries, due to its excellent mechanical, thermal, and electrical characteristics. The carbon fiber market is expected to grow 6.3% year after year until 2016. [1-4].
However, due to the high cost of petroleum-based precursors and their associated processing costs, carbon fiber remains a specialty product and as such has been limited to use in high-end aerospace, sporting goods, automotive, and specialist industrial applications [5-7].
The price of carbon fibers is primarily driven by its manufacture. Within the manufacturing process, petroleum-based precursors account for just over half the cost of the finished carbon fibers, and so across the industry, manufacturing costs are dominated by the high cost of carbon fiber precursor materials [8]. Carbon fibers are usually produced from polyacrylonitrile (PAN), pitch, and rayon materials. Industrial users of carbon fibers generally utilize commercially popular PAN-based carbon fiber [9-11]. However, the high cost of PAN precursors, which make up 43% of the carbon fiber manufacturing cost, limits its utilization in general performance grade applications. [12-14].
To date, investigations into the manufacture of low-cost carbon fiber have been limited to a small number of organizations owing to the magnitude of the effort required, a limited availability of expertise, and equipment. Approaches involve reducing cost by using lower cost materials, by reducing the cost of processing, or by a combination of the two [15-19].
Polyolefins (PE, PP, etc.) can easily be used to produce fibers at low cost, and have been applied as industrial fiber. Also, polyolefin has a high carbonization ratio in the carbonization process, and it can be melt spun. Because of these advantages, polyolefin is promising for the manufacture of a low-cost carbon fiber. Low-density polyethylenes (LDPE) are attractive as carbon precursors due to their high carbon content and ease of manufacture. Also, highly oriented LDPE, with extraordinary physical and mechanical properties, are available today. However, being thermoplastic, they melt at a fairly low temperature, losing their form. These precursors have to be stabilized by introducing cross-links into the polymer structure, so that they can withstand the high carbonization temperatures [20-22].
LDPE are usually cross-linked in four kind different ways: by energy radiation, peroxide, silane coupling agents, and sulfonation cross-linking [23]. Among the four cross-linking methods used, the sulfonation cross-linking method has the advantage of high cross-linking density. With sulfonation crosslinking it is possible to easily adjust the cross-linking density by temperature control. The sulfonating agent in this process is typically sulfuric acid, chlorosulfonic acid, or a combination thereof. The sulfuric acid cross-linking method uses concentrated sulfuric acid (90 wt. % or more). The concentrated sulfuric acid has a strong dehydrating action because it is strongly hygroscopic. In contact with organic material it has the property of absorbing hydrogen and oxygen in the form of water molecules. [24-26].
In the present study, we tried to develop a low price carbon fiber from LDPE-based fibers through sulfonation cross-linking. Various analytical techniques were utilized to investigate the morphology and thermal properties of the precursors and final carbonized fibers. Finally, an exploration of the properties of the carbonized fibers demonstrated the increased carbon yield and clearly fibrous form of the LDPE-based fibers.
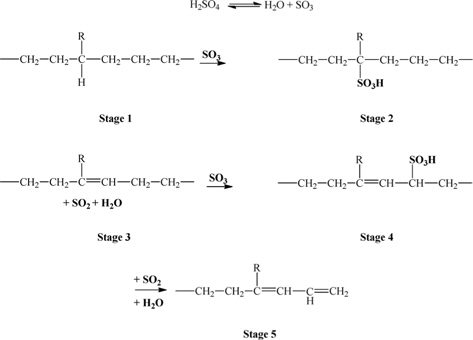
Concentrated sulfuric acid (H2SO4, Daejung Chem, Korea) and low-density polyethylene (LDPE, LG Chem, Korea) were obtained for the experiments. The cross-linking of LDPE was carried out in hot sulfuric acid. The concentrated (98%) sulfuric acid was heated to a temperature of 130-170℃. After treatments for a predetermined length of time and temperature, the LDPE was taken out, washed thoroughly in distilled water, and then dried in a constant temperature oven at 60℃ for 24 h. The completely cross-linked fibers were carbonized in a vertical quartz tubular furnace at 900℃ for 30 min (nitrogen purging) with the heating rate of 5℃/min at slight weight tension. The reaction mechanism of the sulfuric acid with LDPE is illustrated in Fig.1 [27].
The effects of the temperature characteristics of the crosslinking processes were studied using thermal analyses, such as differential scanning calorimetry (DSC-60, SHIMADZU, Japan) and thermogravimetric analysis (TGA-50, SHIMADZU, Japan). DSC analysis of the heating and cooling rate of 10℃/ min was used with a temperature range from 30 to 300℃. The sample was purged with nitrogen gas to maintain an insert environment, at a flow rate of 20 ml/min. And, TGA analysis of all samples were examined under a pure nitrogen atmosphere. Each of the samples were heated from 30℃ up to 900℃ at a rate of 10℃/min. The surface morphologies of the carbonized fibers were observed by scanning electron microscope (AIS2000C, Seron Tech, Korea). SEM analysis was performed at high voltage (15 kV) after gold coating.
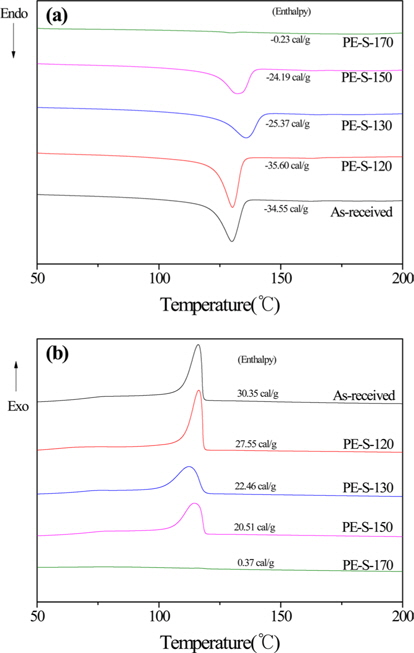
Fig. 2 shows the DSC curves of the LDPE precursor, and LDPE fiber cross-linked by sulfuric acid at different cross-linking temperatures. It shows almost no cross-linking of the LDPE fibers under 120℃ reaction temperature. But, the Tm and Tc enthalpy of the fibers decreased as the cross-linking temperature increased to at least 130℃. The Tm enthalpy of the fibers decreased as the cross-linking temperature increased. That is due to the decomposition and change of crystal structures from extended to folded morphology. The Tm and Tc temperature of the extended-chain polyethylene was higher than that of folded-chain polyethylene. The endotherm points and exotherm points disappeared after sufficient sulfonation due to the penetration and diffusion of sulfuric acid molecules from the surface of the crystals to their center. The DSC peak of sufficiently cross-linked LDPE disappeared by increased cross-linked density at 170℃.
3.2. Thermogravimetric analysis
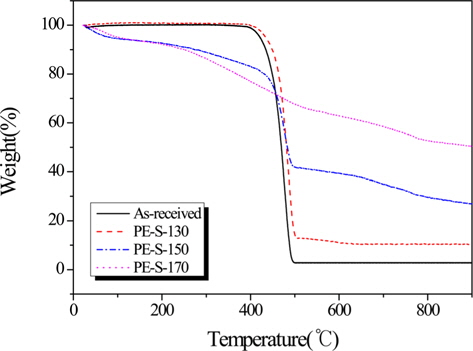
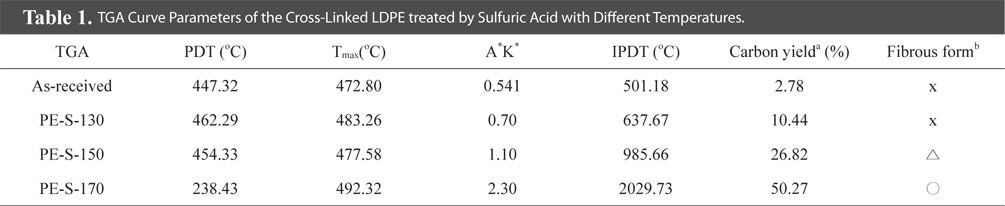
Fig. 3 shows the increasing weight retention with increasing cross-linked temperature. And, Table 1 lists the TGA results for LDPE fiber cross-linked by sulfuric acid with different temperatures. Decomposition temperatures for the all LDPE fibers were about 500℃ except PE-S-170. The thermal stability, based on the polymer decomposition temperature (PDT) and integral procedural decomposition temperature (IPDT), rose with increasing sulfonation temperature. As the crosslinking temperature was increased, the carbonization yield was increased. The cross-linking reaction strengthened decomposition stability and heat exhaustion, resulting from the most densely cross-linked structure at 170℃. This also enhanced gel-structures in the LDPE, resulting in high carbonization yields. From Table 1, PE-S-170 showed the best weight retention (50.27%), among all the cross-liked samples.
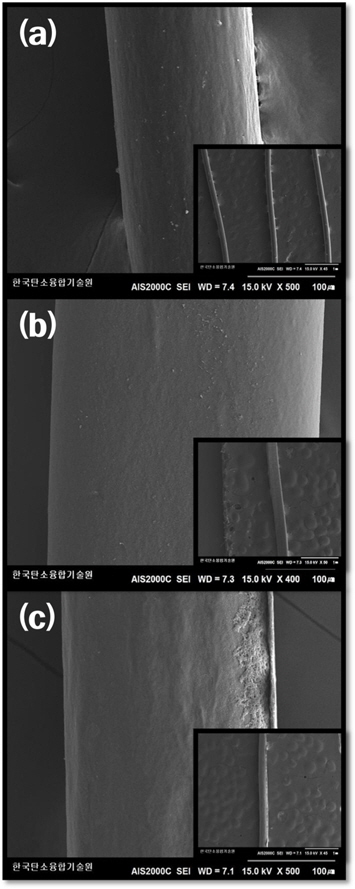
3.3. Surface morphology analysis
The surface morphologies of all the LDPE fibers were observed by scanning electron microscope (SEM). SEM images of the LDPE fibers are shown in Fig. 4 (a). As seen in Figure 4, the morphology of the fibrous surfaces of all specimens was similar to each other. On closer scrutiny of this figure, it can also be found that the fibrous surface morphology differs in roughness. The fibrous form of the samples can be clearly observed as carbonized fibers from Fig. 4 (c). From SEM and TGA results, it can be assumed that proper carbon yield (over 50%) can lead to good fiber shape after the carbonization.
In this work, LDPE was used as a precursor and cross-linked by sulfuric acid to obtain a high degree of carbonization. Cross-linking behaviors of the samples were clearly observed with increasing reaction temperatures in sulfuric acid from DSC results. From the TGA results, the residues of the samples increased gradually up to 150℃ of reaction temperature, then were dramatically enhanced at 170℃. This result indicates that the cross-linking reaction of the LDPE in sulfuric acid can occur at low temperature, but high temperature (over 170℃) can radically accelerate the cross-linking reaction. The final residue of ‘PE-S-170’ was over 50%. In addition, the fibrous states of the samples were clearly observed to be carbonized fibers from the SEM results. From the results, LDPE can be a potentially advantageous material as a carbon fiber precursor.