Light emitting conjugated polymers have attracted a great deal of attention because of their interesting electroluminescent properties and their potential to function as the active emitting layer in light-emitting diodes (LED). Polymeric LEDs have many advantages for the development of a large-area visible lightemitting display because of the good processability, low operating voltage, fast response time, and color tunability over the full visible range by the control of the HOMO-LUMO band gap of the emissive layer [1-5]. Most of the research in the field of polymerbased electroluminescent devices has been focused on mainchain conducting polymers such as poly(p-phenylenevinylene) (PPV) [6,7], poly(p-phenylene) (PPP) [8,9], poly(thiophene) [10], and poly(fluorene) [11,12]. Among them, the most promising and extensively studied group of polymers is poly(p-phenylenevinylene) (PPV) and its derivatives, on account of their good device performance.
It has also been known that recombination of electrons and holes injected from cathode and anode produce emissions in the luminescent polymer layer of the LEDs. Therefore, balanced charge injections from both electrodes are important for high device efficiencies [13,14]. It was reported that the HOMO-LUMO energy levels could be lowered for balanced charge injections by the introduction of electron-withdrawing substituents onto the conjugated main chain of the polymer. The electron withdrawing groups such as halide, cyano, trifluoromethyl, or (methylsulfonyl) phenyl were introduced on the arylene rings of PPV derivatives [15-19].
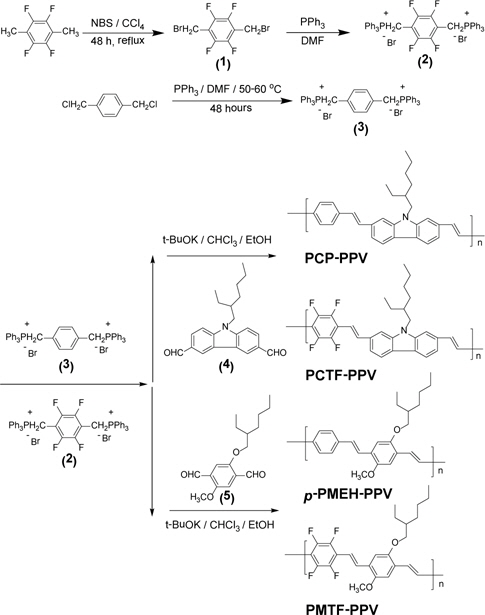
In this paper, we report on the syntheses and light-emitting properties of 2,3,5,6-tetrafluorophenyl containing poly(p-phenylenevinylene) related alternating copolymers. High electron affinity (tetrafluorophenyl) and hole-transporting (carbazole) units are incorporated together into the conjugated main chain. To the best of our knowledge, this research is the first to report on tetrafluorophenyl and carbazole groups insertion onto the PPV backbone. All the synthetic routes and polymer structures are shown in Scheme 1.
Materials. 1,2,4,5-Tetrafluoro-3,6-dimethylbenzene, 1,4-bis(chloromethyl)benzene, carbazole, 4-methoxyphenol, 2-ethylhexylbromide, N-bromosuccinimide (NBS), triphenylphosphine (PPh3), potassium t-butoxide, and pyridinium chlorochromate from Aldrich Chemical Co. were used without any further purification. N,N-Dimethylformamide (DMF), 1,4-dioxane, acetic anhydride, sodium hydride (NaH), tetrahydrofuran (THF), ethanol, methanol, and chloroform from Junsei Chemical Co. were used without purification.
Instrumentation. The 1H NMR spectrum was recorded with a Bruker AMX 300 MHz spectrometer. The UV-visible spectra of the PCP-PPV, PCTF-PPV, p-PMEH-PPV and PMTF-PPV were measured on a Shimadzu UV-3100S. The FT-IR spectra were measured by an EQUINOX 55 spectrometer. The thermogravimteric analyses (TGA) and differentional scanning calorimetry (DSC) measurements of polymers were performed under nitrogen atmosphere at a heating rate of 10℃/min with Dupont 9900 analyzers. Cyclic voltammetry was performed on an AUTOLAB/PGSTAT12 model system with a three-electrode cell in a solution of Bu4NBF4 (0.10 M) in acetonitrile at a scan rate of 50 mV/s. Polymer films were coated on a square Pt electrode (0.50 cm2) by dipping the electrode into the corresponding solutions and then drying them in argon. A Pt wire was used as the counter electrode and a Ag/AgNO3 (0.10 M in acetonitrile) electrode as the reference electrode. The photoluminescence spectra of the polymers were obtained by using a Spex Fluorolog-3 (model FL3-11) spectrofluorometer. EL spectra of an LED device were measured with a Minolta CS-1000 spectroradiometer. Luminance-current-voltage (L-I-V) characteristics were recorded using a programmable current/voltage source (Keithley 238) and a luminance meter (Minolta LS-100).
Diphosphonium Salts Monomers. 1,4-Bis(bromomethyl)- 2,3,5,6-tetrafluorobenzene (1). A 2.0 g (11.0 mmol) of 2,3,5,6-tetrafluoro-1,4-dimethylbenzene was dissolved in about 60 mL of carbon tetrachloride (CCl4) with 4.3 g (24.0 mmol) of N-bromosuccinimide (NBS) in a 100 mL two-necked flask. A small amount of benzoyl peroxide was added as an initiator. The reaction mixture was refluxed at 80~90℃ for 48 hours under nitrogen atmosphere. The completion of the reaction was indicated by the appearance of succinimide on the surface of the reaction solution. The solution was extracted with methylene chloride and water after the filtration of succinimide. The solvent was then removed by distillation to give 2.0 g (59.8%) of white solid. Mp: 143℃. 1H-NMR (CDCl3, ppm): δ 4.49 (s, 4H, -CH2Br). Anal. Calcd for C8H4Br2F4: C, 28.60; H, 1.20. Found: C, 28.61; H, 1.27.
1,4-Tetrafluorobenzenebis(triphenylphosphoni um bromide) (2). A solution of 3.5 g (10.0 mmol) of 1,4-bis(bromomethyl)-2,3,5,6-tetrafluorobenzene and 6.3 g (24.0 mmol) of triphenylphosphine (PPh3) in 50 mL N,N-dimethylformamide (DMF) was stirred and heated to 50~60℃ for 24 hours. The resulting mixture was poured into diethyl ether. After filtration and vacuum drying, the diphosphonium salt monomer was obtained as a white powder. The product yield was 7.5 g (87.2%). Mp: 347℃. 1H-NMR (CDCl3, ppm): δ 7.80 (m, 30 H, Ar H), 5.34 (d, 4H, -PCH2-). Anal. Calcd for C44H34Br2F4P2: C, 61.45; H, 3.99. Found: C, 60,98; H, 4.03.
1,4-Xylenebis(triphenylphosphonium bromide) (3). A solution of 5 g (29.0 mmol) of α,α’-dichloro-p-xylene and 18.73 g (71.0 mmol) of PPh3 in 60 mL N,N-dimethylformamide (DMF) was stirred and heated to 70℃ for 24 hours. When the reaction was complete, the solution was poured into diethyl ether. After filtration and vacuum drying, a white solid was obtained. The crude product was dissolved in methylene chloride and precipitated in diethyl ether. The precipitate was collected on a Buchner funnel, washed with 100 mL of acetone, and vacuum dried. The product yield was 15.1 g (74.4%). Mp: 395℃. 1H-NMR (CDCl3, ppm): δ 7.67 (m, 30 H, Ar H), 6.89 (s, 4H, Ar H), 5.41 (d, 4H, -PCH2-). Anal. Calcd for C44h38Br2P2: C, 67.02; H, 4.87. Found: C, 67,38; H, 4.83.
Dialdehyde Monomers. 9-(2-Ethyl-hexyl)carbazole-3,6-dicarbaldehyde (4). It was synthesized by a procedure adapted from the literature. We have used this adapted procedure in our previous work [7].
5-(2-Ethylhexyloxy)-2-methoxybenzene-1,4-dicarbaldehyde (5). It was synthesized by a procedure adapted from the literature. We have used this adapted procedure in our previous work [20].
Polymerization of PCP-PPV, PCTF-PPV, p-PMEH-PPV and PMTF-PPV.
PCP-PPV. A solution of 1.0 g (1.4 mmol) 1,4-xylenebis(tripheny lphosphonium bromide) (3) and 0.47 g (1.4 mmol) of 9-(2-ethylhexyl)carbazole-3,6-dicarbaldehyde (4) was prepared. To 10 mL ethanol, 0.79 g (7.0 mmol) of potassium t-butoxide was dissolved and this base solution was carefully dropped on the solution of monomers. The following synthetic procedures were performed in the same manner for PMTF-PPV. The polymer yield was 0.24 g (42.1%). 1H-NMR (CDCl3, ppm): δ 8.61-6.55 (br m, 14H, Ar H of carbazole and vinylic H), 4.12 (br s, 2H, -NCH2-), 1.56-1.01 (br m, 9H), 0.92-0.81 (br m, 6H). FT-IR (KBr pellet, cm-1) 960 (trans HC=CH). Anal. Calcd for (C30H31N)n: C, 88.83; H, 7.72. Found. C, 87.56; H, 7.68.
PCTF-PPV. A solution of 1.0 g (1.2 mmol) of 1,4-tetrafluorobe nzenebis(triphenylphosphonium bromide) (2) 0.39 g (1.2 mmol) of 9-(2-ethylhexyl)carbazole-3,6-dicarbaldehyde (4) in 10 mL chloroform was prepared. To 10 mL ethanol, 0.67 g (6.0 mmol) of potassium t-butoxide was dissolved and this base solution was carefully dropped on the solution of monomers. The following synthetic procedures were performed in the same manner for PMTF-PPV. The polymer yield was 0.35 g (61.4%). 1H-NMR (CDCl3, ppm): δ 8.61-6.81 (br m, 10 H, Ar H of carbazole and vinylic H), 4.07 (br s, 2H, -NCH2-), 1.36-0.92 (br m, 9H), 0.91-0.83 (br m, 6H). FT-IR (KBr pellet, cm-1) 962 (trans HC=CH). Anal. Calcd for (C30H27F4N)n: C, 75.44; H, 5.71. Found: C, 74.87; H, 5.46.
p-PMEH-PPV. A solution of 1.0 g (1.4 mmol) 1,4-xylenebis( triphenylphosphonium bromide) (3) and 0.41 g (1.4 mmol) of 5-(2-ethylhexyloxy)-2-methoxybenzene-1,4-dicarbaldehyde (5) in 10 mL chloroform was prepared. To 10 mL ethanol, 0.79 g (7.0 mmol) of potassium t-butoxide was dissolved and this base solution was carefully dropped on the solution of monomers. The following synthetic procedures were performed in the same manner for PMTF-PPV. The polymer yield was 0.32 g (62.8%). 1H-NMR (CDCl3, ppm): δ 7.50-6.65 (br m, 10H, Ar H), 3.92 (br s, 3H, -OCH3), 3.43 (br s, 2H, -OCH2-), 1.80-1.23 (br m, 9H), 1.12-0.82 (br m, 6H). FT-IR (KBr pellet, cm-1) 963 (trans HC=CH). Anal. Calcd for (C25H30O2)n: C, 82.81; H, 8.36. Found. C, 82.10; H, 8.10.
PMTF-PPV. A solution of 1.0 g (1.2 mmol) of 1,4-tetrafluorob enzenebis(triphenylphosphonium bromide) (2) and 0.35 g (1.2 mmol) of 5-(2-ethylhexyloxy)-2-methoxybenzene-1,4-dicarbaldehyde (5) in 10 mL chloroform was prepared. To 10 mL ethanol, 0.67 g (6.0 mmol) of potassium t-butoxide was dissolved and this base solution was carefully dropped on the solution of monomers. After 48 hours, the polymer product was precipitated from methanol. The crude polymer was filtered and then purified by using Soxhlet extractor in methanol for three days. The polymer yields were 0.41 g (80.3%). 1H-NMR (CDCl3, ppm): δ 7.81-6.67 (br m, 6H, Ar H and vinylic H), 3.82 (br s, 3H, -OCH3), 3.50 (br s, 2H, -OCH2-), 1.78-1.33 (br m, 9H), 0.97-0.89 (br m, 6H). FT-IR (KBr pellet, cm-1) 967 (trans HC=CH). Anal. Calcd for (C25H26F4O2)n: C, 69.10; H, 6.04. Found: C, 68.59; H, 6.01.
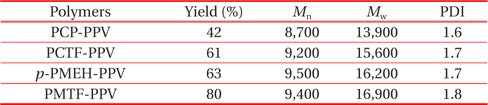
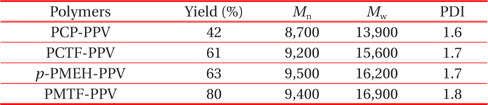
All of the synthesized polymers were highly soluble in common organic solvents such as tetrahydrofuran, chloroform, methylene chloride, and 1,2-dichloroethane. They could be spincast onto various substrates to give highly homogeneous thin films without heat treatment. The polymerization results of all synthesized polymers are summarized in Table 1. The numberaverage molecular weight (Mn) and the weight-average molecular weight (Mw) of the polymers, determined by gel permeation chromatography using polystyrene standards, were in the range of 8,700~9,500 and 13,900~16,900 with a polydispersity index (PDI) of 1.6-1.8, respectively.
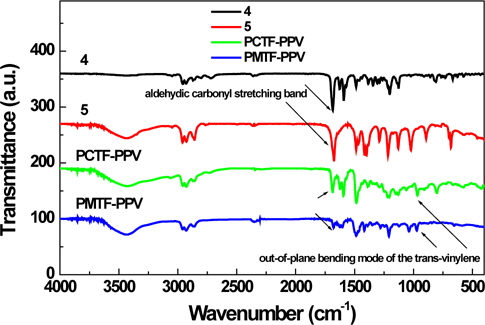
Figure 1 shows the typical FT-IR spectra of tetrafluorophenyl containing PCTF-PPV and PMTF-PPV. Compared to that of corresponding dialdehyde monomers (4 and 5), the intensities of aldehydic carbonyl stretching bands are decreased. In addition, PCTF-PPV and PMTF-PPV show weak but clear absorption peaks at about 965 cm-1 corresponding to the out-of-plane bending mode of the trans-vinylene bonds. This proves the formation of vinylene double bond and consequently the polymerization reactions have been successful [6,20].
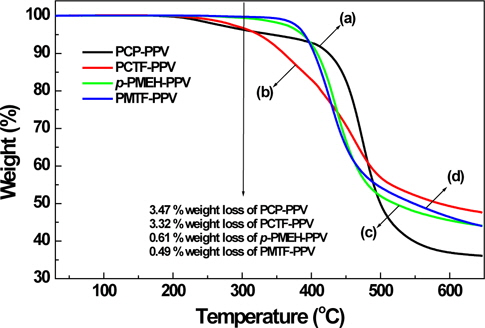
The thermal properties of the synthesized polymers were evaluated by means of TGA under nitrogen atmosphere as shown in Figure 2. All synthesized polymers showed good thermal stability up to 300℃ in TGA thermograms. 5-(2-Ethylhexyloxy)-2-methoxybenzene containing p-PMEH-PPV and PMTF-PPV polymers exhibited better thermal stability than carbazole containing PCP-PPV and PCTF-PPV polymers. This result comes from the stability of the phenyl group than that of the carbazole group on the polymer main chain. In addition, tetrafluorophenyl containing PCTF-PPV and PMTF-PPV seems to have less enhanced thermal stability than the polymers (PCP-PPV and p-PMEH-PPV) that do not have any fluoro groups in the polymer structure. PMTF-PPV showed the highest thermal stability among the polymers. The decomposition temperature (Td, 5% weight loss) was found at about 386℃.
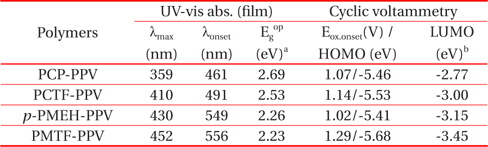
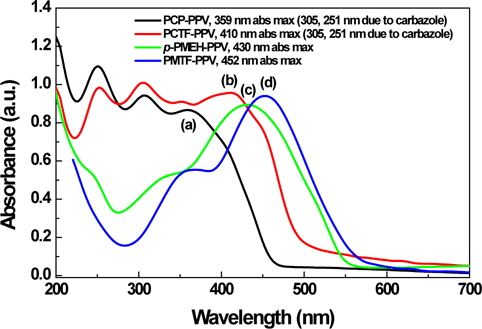
Figure 3 shows the UV absorption spectra of PCP-PPV, PCTFPPV, p-PMEH-PPV and PMTF-PPV in a thin film on a quartz plate. The carbazole containing alternating copolymers, PCP-PPV and PCTF-PPV showed more blue-shifted absorption maxima than p-PMEH-PPV and PMTF-PPV. The maximum absorption wavelengths of PCP-PPV and PCTF-PPV are 359 and 410 nm, respectively. This means that carbazole linkages could more effectively reduce the conjugation length than phenyl linkages of p-PMEH-PPV and PMTF-PPV. The characteristic absorption peaks of PCP-PPV and PCTF-PPV at 305 and 251 nm are due to the carbazole unit in the copolymer main chain.
The maximum absorption wavelengths of p-PMEH-PPV and PMTF-PPV are 430 and 452 nm, respectively. Tetrafluorophenyl containing PMTF-PPV showed a maximum red-shifted absorption of about 22 nm compared to non-fluoro containing p-PMEH-PPV. In the case of carbazole containing PCP-PPV and PCTF-PPV polymers, the tetrafluorophenyl group that has PCTFPPV also exhibited an absorption maximum at a longer region of almost 50 nm, compared to the no fluoro group containing PCP-PPV. These results mean that the fluoro group substitution could effectively increase the conjugation length of the polymer.
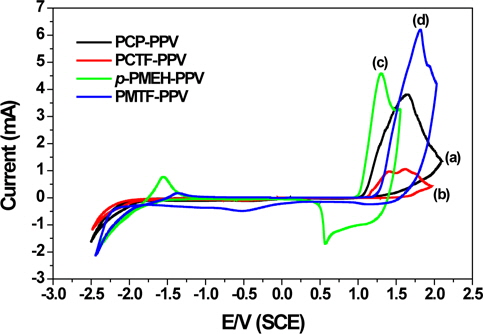
Cyclic voltammetry (CV) is an easy and effective approach for measuring redox reversibility, reproducibility, and stability of polymer films on electrodes. In addition, it can be utilized to simultaneously evaluate both the HOMO and LUMO energy levels and the band gap of a polymer. CV was performed on a solution of 0.1 M tetrabutylammonium tetrafluoroborate (Bu4NBF4) in acetonitrile with a scan rate of 50 mV/s at room temperature. A platinum electrode (~0.5 cm2 ) was coated with a thin polymer film (PCP-PPV, PCTF-PPV, p-PMEH-PPV, or PMTF-PPV) and used as the working electrode. Platinum wire was used as the counter electrode, and an Ag/0.10 M AgNO3 electrode was used as the reference electrode. The cyclic voltammograms from PCP-PPV, PCTF-PPV, p-PMEH-PPV, and PMTF-PPV are displayed in Fig. 4.
The onset potentials (Eox) of p-doping are used to determine the HOMO energy levels of the conjugated polymers by means of an empirical relationship proposed by Leeuw et al [21]. This relationship connects the solid-state IP (HOMO) to the Eox using the relation IP (EHOMO) = -(Eox + 4.39) (eV) where EHOMO is the HOMO energy level below the vacuum. The LUMO levels between the HOMO energy levels and the optical band gaps were also calculated, the LUMO energy levels of PCP-PPV, PCTF-PPV, p-PMEH-PPV, and PMTF-PPV were found to be -2.77, -3.00, -3.15 and -3.45 eV, respectively. All data are summarized in Table 2. Tetrafluorophenyl containing PCTF-PPV and PMTF-PPV have significant more negative [IP(HOMO)] values than their corresponding non-fluoro group containing PCP-PPV and p-PMEH-PPV. This result means that it is difficult to inject holes from ITO into the polymers in organic light-emitting diode (OLED) devices. Usu ally PPV derivatives have major hole characteristics in EL devices, and an imbalance between the injected hole and electron, with an air-stable metal cathode. However, by incorporating the tetrafluorophenyl group into the polymer main chain of PCTFPPV and PMTF-PPV, the retarded hole injection could show more balanced hole and electron injection than other PPV derivatives. Therefore, low operating voltages and improved EL device performances might be expected using this tetrafluorophenyl group that has PCTF-PPV and PMTF-PPV.
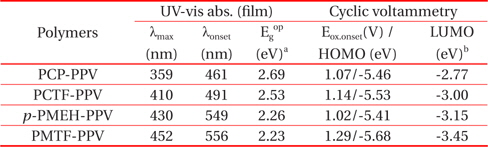
The PL spectra showed drastic changes of emission color because of the carbazole and tetrafluorophenyl group linkages. The PL spectra of PCP-PPV, PCTF-PPV, p-PMEH-PPV, and PMTF-PPV polymer thin films coated on a quartz plate were shown in Fig. 5.
The maximum emission peak of the carbazole containing PCPPPV was found at about 500 nm with a shoulder peak at 478 nm. The PCP-PPV polymer shows an almost sky-blue color. By substituting a tetrafluorophenyl group to PCP-PPV, the emissions of PCTF-PPV shifted considerably; it shifted shifted about 20 nm to the longer wavelength. This clearly shows that fluoro group substitution can extend the conjugation length of the polymer. For the case of p-PMEH-PPV, the maximum emissions were found at 550 nm. Compared to the carbazole containing PCP-PPV, almost 50 nm is red-shifted. This means that the dialkoxy substituted phenyl group could more effectively facilitate the π-conjugation than the carbazole group. In addition, by fluoro group substitution, PMTF-PPV showed far more red-shifted emission at 580 nm than p-PMEH-PPV. The emission maximum of PMTF-PPV is almost similar the to that of well know MEH-PPV polymer [20]. We found that the tetrafluorophenyl linkages effectively extend the conjugation length of the polymers. Therefore, the tetrafluorophenyl group containing PCTF-PPV and PMTF-PPV polymers showed more red-shifted emissions than corresponding nonfluoro group containing PCP-PPV and p-PMEH-PPV.
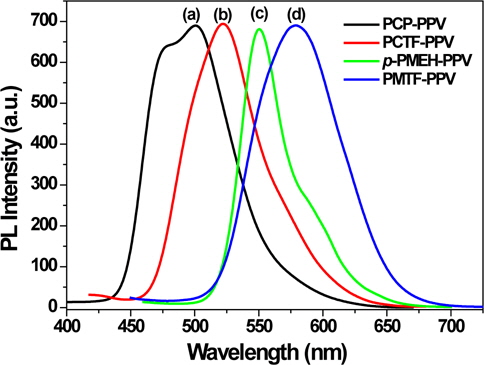
To investigate the potentials of synthesized polymers as an emissive layer in LED devices, a single-layer LED was fabricated by using ITO as the anode and Al as the cathode, respectively. The PCP-PPV, PCTF-PPV, p-PMEH-PPV and PMTF-PPV polymers were deposited onto indium-tin oxide-covered glass substrates by spin-casting the soluble polymers in chloroform. The spincasting technique yielded uniform films with thicknesses of about 70 nm. The Al electrode (cathode) was then evaporated onto this in vacuo with a thickness of about 120 nm. The EL spectra of PCP-PPV, PCTF-PPV, p-PMEH-PPV and PMTF-PPV polymer films are shown in Fig. 6. The PCP-PPV and PCTF-PPV polymer films showed maximum EL emissions at 507 and 524 nm, respectively. These emissions were both slightly red-shifted to that of the PL spectra of PCP-PPV and PCTF-PPV.
The p-PMEH-PPV exhibited far more red-shifted EL emission at 556 nm than the carbazole containing PCP-PPV polymer. This is almost similar behavior to that of PL emissions of PCP-PPV and p-PMEH-PPV. As we observed these trends in UV-visible absorptions and PL emissions, π-conjugation length could be controlled by the carbazole and dialkoxy-substituted phenyl group. Surprisingly, tetrafluorophenyl containing PMTF-PPV exhibited extremely red-shifted EL emission at 616 nm. It is about 60 nm red-shifted in comparison to the EL emission of p-PMEH-PPV. It almost looks red to the naked eye. The reason why PMTF-PPV emits far more red-shifted EL is not fully understood. However, the effect of the tetrafluorophenyl group is obvious. We can conclude that the insertion of tetrafluorophenyl on the polymer main chain can be a good approach to extend the π-conjugation length and to get the emission at longer wavelength regions.
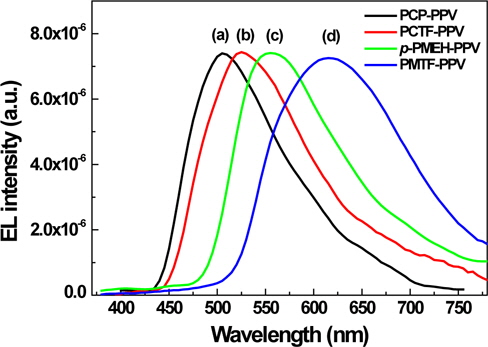
Figure 7 shows the current vs. voltage characteristics measured from the LED devices of synthesized polymers. The forward current increases with increasing forward bias voltage for all devices. Interestingly, tetrafluorophenyl containing PCTF-PPV and PMTF-PPV exhibit much lower threshold voltages than their corresponding non-fluoro group containing PCP-PPV and p-PMEH-PPV polymer.
Clearly, these data show that the insertion of the tetrafluorophenyl group into the polymer could bring a more balanced hole and electron injection in other words, the low threshold voltages in EL devices. The hole transporting carbazole and electron transporting tetrafuorophenyl that has PCTF-PPV showed the lowest threshold voltage at about 10 V compared to the other polymers. Further device optimizations and electrical characterizations of the synthesized polymers are in progress by replacing the cathode electrode with LiF/Al or Ca/Al or by the insertion of a PEDOT layer as a hole injection layer to increase the EL efficiency of the synthesized polymers.