The journey of liquid crystals began in 1888, when F.Reinitzer reported his famous article on cholesteric liquid crystal [1]. Liquid crystals are a class of materials that possess the properties of liquid as well as crystal. They are classified on many bases. According to their molecular arrangement they are classified as Nemetic, Cholesteric and Smectic. Cholesteric liquid crystals possess several unique properties, such as super molecular, periodic, helical structure due to the chirality of the molecules, 100% selective reflection of circularly polarized light, ability to control selective reflection wavelength and a changing electric and magnetic field [2,3]. Cholesteric liquid crystals have useful applications in reflective displays, diffusive reflectors, and reflective polarizers because of their unique properties [4-6].
Any conventional laser needs the presence of positive feedback and the presence of an active medium to operate. Usually resonator mirrors are used as feedback elements. But there is a class of lasers in which external feedback is absent and the active medium itself acts as a feedback element. For this purpose, the active medium must have a spatial periodic structure. In this respect, cholesteric liquid crystals are the materials that can be used as active materials in distributed feedback lasers. In the present paper, we discuss the results of investigations into the lasing characteristics of dye doped cholesteric liquid crystals.
2. ELECTRO OPTICAL PROPERTIES OF DYE DOPED CHOLESTERIC LIQUID CRYSTALS
Cholesteric liquid crystals naturally form helical structures with the helical pitch in an optical wavelength range [7]. The dye and cholesteric liquid crystal molecules are together subjected to the electric field action, which produces a collective movement that leads to changes in molecular arrangement, notably the applied electric field direction. As a result of the applied field, the helical molecular arrangement axis is perpendicular to the electric field direction and then produces an increase in the helical structure pitch [8,9].
A cholesteric liquid crystal can be considered as a one-dimensional photonic crystal with a refractive index that is regularly modulated along the helix axis because of the particular arrangement of the molecules. The result is that the propagation of light is suppressed for a particular range of wavelength [10]. Liquid crystals are found to be quite absorptive in the UV region. In the visible and near infrared regions there are fewer absorption bands and, hence, liquid crystals are quite transparent in these regions. It has been observed that doping dyes into liquid crystals significantly increase their optical responses due to increased absorption in the visible region.
3. LASING IN PURE CHOLESTERIC LIQUID CRYSTAL
Cholesteric liquid crystals spontaneously form a one-dimensional periodic structure and their molecules have a high birefringence. Hence, cholesteric liquid crystals give a periodic modulation for the refractive index and can be regarded as a one-dimensional photonic crystal. Therefore, planar cholesteric liquid crystals can be used as mirrorless distributed feedback resonators. The fluorescent emission in these materials is suppressed in the reflection band and is enhanced at the band edges. This enhancement and distributed feedback effect can give rise to low threshold mirror-less lasing at the band edge in various liquid crystal materials [12-21].
Cholesteric liquid crystals self-organize in a helical structure and selectively reflect light. The selective reflection band (photonic band gap) is centered at the wavelength λ=np where p is the helical pitch of the chiral liquid crystal and n = (ne+no)/2 is the average refractive index of the cholesteric planes. If the helical pitch is altered, the color of the sample and the position of the selective reflection band changes.
The possibility of lasing in cholesteric liquid crystal was first proposed by Goldberg et a1. in 1973 [22]. Kukhtarev proposed the idea and developed a theory of cholesteric liquid crystal distributed feedback lasers in 1978 [23]. Ilchishin et al. performed the first experimental observation of the lasing action in cholesteric liquid crystal . in 1980 [24]. In the pure system, the cholesteric liquid crystal acts both as the cavity and the active medium.
4. LASING IN DYE DOPED CHOLESTERIC LIQUID CRYSTAL
By doping active dyes in the cholesteric liquid crystals, the spontaneously emitted fluorescence is suppressed within the gaps and is enhanced at band edges. Lasing in dye-doped systems was demonstrated by Kopp et al [25]. and by Taheri and Palffy-Muhoray et al. [26] in the visible range under picoseconds pulse excitation at 532 nm. Dye-doped cholesteric liquid crystals are known as materials for compact, low threshold, distributed feedback lasers [26,28] emitting narrow band light within the whole visible range [29].
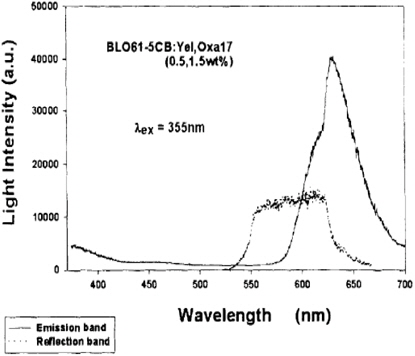
E. Alvarez et al. observed mirror-less lasing in cholesteric liquid crystal samples doped with two dyes benzofuran and oxazine under UV pulsed excitation at 355 nm [30]. The emission and excitation spectra of the constituents had been studied in the 300-700 nm range. The excitation spectra gave proof of active transfer from the host cholesteric liquid crystal to the benzofuran dye and from the benzofuran dye to the oxazine dye. The benzofuran dye was found to act as an impedance transformer facilitating energy transfer from the host cholesteric liquid crystal to the lasing oxazine dye.
Figure 1 shows the unpolarized emission spectrum of the cholesteric liquid crystal-benzofuran-oxazine system below the lasing threshold with pulsed excitation at 355 nm. The small peak in the emission at 385 nm was assumed due to the de-excitation of the host cholesteric liquid crystal molecules from the lowest excited state to the ground state.
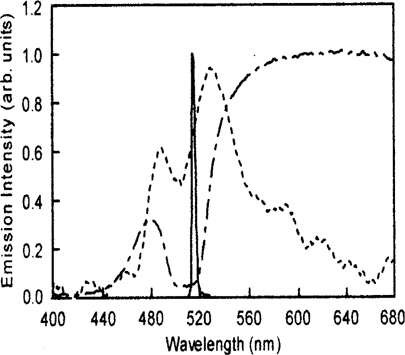
Yoichi Takanishi et al. observed lasing with a low threshold in a cholesteric liquid crystal doped with polymeric dyes with the transition dipole moment parallel to the local director of the cholesteric liquid crystals host. It was found that lasing always occurs at the lower-energy edge of the photonic gap [31].
Figure 2 indicates an emission spectrum above the threshold energy (Ethreshold) at 60℃. A narrow and sharp emission peak appeared just at the low-energy edge of the photonic stop band, which proved that the emission above the Ethreshold is a laser emission caused by the photonic effect of the helix of the cholesteric liquid crystal.
Andro Chanishvili et al. reported the results of photo tunable lasing in dye-doped cholesteric liquid crystals. Photoexcitation of dye doped cholesteric liquid crystal films give rise to laser emission in the violet-UV range. Control of the structure of the chiraldopant driven by UV photo transformation was exploited in order to obtain a permanent variation of the cholesteric pitch. Laser emission wavelength tuning was established with the help of photoinduced shifting of the selective reflection band of the cholesteric liquid crystals [32].
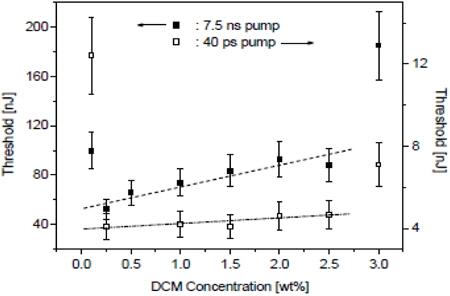
W. Cao et al. studied the lasing thresholds of cholesteric liquid crystal lasers as a function of dye concentration and of sample thickness [33].
Figure 3 shows the threshold dependence on dye concentration. The threshold exhibits similar behavior with both nanosecond and picosecond pump. Thresholds were found significantly higher at both the low and high ends of the concentration range. To overcome losses, the medium has to provide a gain that is greater than the gain threshold. As the gain is proportional to the dye concentration, there can be no lasing if the dye concentration is below the concentration corresponding to the gain threshold.
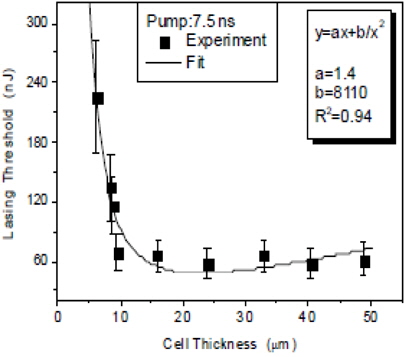
Figure 4 shows the lasing threshold as a function of sample thickness at the fixed dye concentration of 0.5 wt%. It was observed that the threshold decreases to reach a minimum as the cell thickness increases and then gradually increases.
The results showed that the lasing threshold is at its lowest when the dye concentration is in the range of 0.1~3.0 wt%, and the sample thickness is in the range of 5~50 μm. This approach could be useful in the design of cholesteric liquid crystal lasers for device applications.
Svetlana G. Lukishova et al. used fluorescence antibunching from single terrylene molecules embedded in a cholesteric liquid crystal host to demonstrate operation of a room temperature single photon source [34]. One-dimensional photonic band gap micro cavities in planar aligned cholesteric liquid crystals with band gaps from visible to near-infrared spectral regions were fabricated.
Andro Chanishvili et al. reported the observation of a laser emission from a luminescent dye-doped cholesteric liquid crystal excited by another dye-doped cholesteric liquid crystal laser [35]. The idea was based on the cascade of two cholesteric liquid crystal cells containing two different dyes where the emission band of the first overlaps the absorption band of the second. This system of low threshold mirror-less lasers emphasized the main advantages of organic materials for lasing applications and identified a simple laser device.
Tsung-Hsien Lin et al. examined a planar cholesteric cell doped with two laser dyes as a one-dimensional photonic crystal [36]. When photo tunable chiral material was added, the cholesteric liquid crystal was lased at the band edges of the photonic band gap with a tuning range of over 100 nm. Tuning was performed. This involved irradiating the chiral material with UV light with the material undergoing trans-Cis isomerization in the cholesteric liquid crystal film. The tuning range was the visible range from 563 to 667 nm.
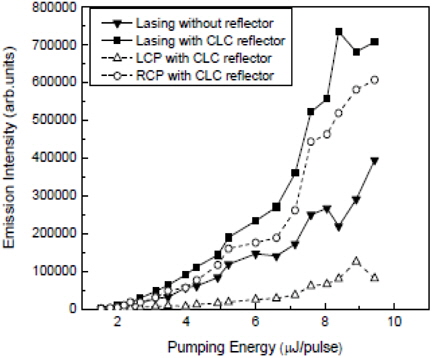
Ying Zhou et al. demonstrated a simple method to double the extracted laser output power in dye-doped cholesteric liquid crystal laser [37]. At an oblique incidence, a metallic mirror or a cholesteric liquid crystal reflector with a reflection band covering the lasing wavelength was placed in close contact with the cholesteric liquid crystal sample. By using a mirror reflector a nearly unpolarized cholesteric liquid crystal laser could be obtained based on incoherent supposition of two orthogonal circularly polarized beams. However, nearly double the laser output could be achieved with a single polarization state for the cholesteric liquid crystal reflector.
Figure 5 shows the pump energy dependent laser emission when a cholesteric liquid crystal reflector is placed in contact with the surface of cholesteric liquid crystal lasing cell. It shows that the total laser intensity is increased by two times and that the circular polarized component on the right dominated.
Yuhua Huang et al. studied the polarization effect of the pulsed pumping laser on the lasing characteristics of the dyedoped right-handed cholesteric liquid crystal at different incident angles [38]. At a small incident angle (<18°), the cholesteric liquid crystal laser performance was independent of the polarization state of the pumping laser. As the incident angle exceeded 18°, the cholesteric liquid crystal laser emission became dependent on the polarization state of the pumping laser. As the incident angle increased, the lasing efficiency of the cholesteric liquid crystal laser was seen to decrease suddenly for those excited by the right-handed circularly polarized or plane polarized laser but decreased slighty for those excited by the left-handed circularly polarized laser. As the incident angle exceeded a 31° lasing phenomenon vanished under the excitation of the righthanded circularly polarized laser. The lasing threshold pumped by the plane-polarized laser was found to be twice as that of the left-handed circularly polarized laser.
Ying Zhou et al. demonstrated a direction controlled linearly polarized laser from a dye doped cholesteric liquid crystal with a metallic mirror coated on the inner side of a glass substrate [39]. It was observed that laser emission became linearly polarized with enhanced energy due to the superposition of two orthogonal polarization states. On analyzing the rotation effect, the polarization direction was found to be proportional to the cholesteric liquid crystal effective birefringence and the cell gap. On the basis of this effect, the temperature and cell gap were used to control the linear polarization direction and the direction tunable cholesteric liquid crystal laser device was realized. This device was highly useful in controlling the polarization.
Ying Zhou et al. also incorporated a metallic mirror reflector into the cholesteric liquid crystal laser on one side so that laser light could only be emitted from one direction and output energy could be enhanced [40]. Two different types of cholesteric liquid crystal lasers with different polarization states were demonstrated by placing the mirror at different substrates. A nearly unpolarized cholesteric liquid crystal laser based on an incoherent combination of two orthogonal circularly polarized beams was obtained with a mirror attached to the outer side of the liquid crystal substrate and a linearly polarized cholesteric liquid crystal laser based on a coherent combination of two orthogonal circularly polarized beams was obtained with a mirror attached at one of the inner sides of the liquid crystal cell.
Yuhan Huang et al. demonstrated a spatially tunable laser emission of the dye doped cholesteric liquid crystal cell using a one-dimensional temperature gradient [41]. Laser emission in the yellow-red spectral range was observed by photo excitation of a dye-doped cholesteric liquid crystal cell device using a frequency-doubled pulsed Nd: yttrium-aluminum-garnet laser. The lasing wavelength was found widely tunable from 577 to 670 nm by changing the position of the dye-doped cholesteric liquid crystal cell with respect to the pumping beam.
Guram Chilaya et al. achieved stability of dye doped cholesteric liquid crystal laser emission from several minutes up to few hours by rotating the liquid crystal cell [42]. The time stability of the laser emission power for rotating cells was investigated. In cells that had plates covered with polyimide and contained uvitex dye the output power did not change for two hours. However, when the Oxazine was used as dye in a cell with polyimide layers, a decrease of the output power was observed for one hour.
Ying Zhou et al. demonstrated an enhanced circularly polarized laser emission. Ying Zhou et al. used the opposite side to the cholesteric helix by the stacked cholesteric polymeric films [43].) Handedness means polarization state (i.e. Left handed or Right handed). The dye-doped right-handed cholesteric polymer film was sandwiched between a mirror and a cholesteric polymer reflector. Due to the stimulated amplification and light recycling effects, the original laser emission from the middle active layer was not only enhanced but also converted to a left-handed circularly polarized emission with high purity.
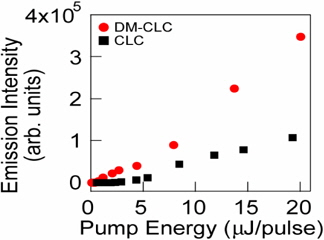
Yuko Matsuhisa et al. demonstrated the low threshold laser action of dye-doped cholesteric liquid crystals using an input circularly polarized light whose direction was the same as the cholesteric helix of the sample at the high-energy band edge of the reflection band [44]. They also demonstrated the laser action of the cholesteric liquid crystal with the dielectric mirrors and found that its output lasing power was much higher and lasing threshold was lower than that of the cholesteric liquid crystal without the dielectric mirrors. In such a device configuration, the band-edge excitation at a high-energy band edge improved the lasing performance for the same circularly polarized pump beam as well as for the opposite one to the cholesteric helix.
Figure 6 shows the emission intensity as a function of the pump energy of the cholesteric liquid crystal with and without dielectric mirrors (DM) for the right handed circular polarized pump beam in which the high-energy band edge coincides with the pumping beam wavelength. It shows that the output lasing power was enhanced 3.4 fold when the dielectric mirrors were added.
N. M. Shtykov et al. showed that it was possible to amplify the weak emission intensity of microlasers based on cholesteric liquid crystals by thin planar layers of both isotropic dye solutions and dye doped nematic liquid crystals [45]. Very high gain index values and absolute amplification of about 15 had been demonstrated. The anisotropy of gain in nematics allows the field control of the light amplification by an electric field. The planar amplifiers using nematic liquid crystals could easily be integrated within single planar devices together with distributed feedback cholesteric liquid crystal microlasers. In such a hybrid structure, the intensity of the cholesteric liquid crystal microlaser was amplified six-fold.
V. M. Pergamenshchik et al. has shown that the temperaturedependent selective reflection spectra in the cholesteric phase manifest the behavioral features of non-mesogenic dopants in liquid crystal matrices [46]. The formation of supramolecular aggregates of anthraquinone dyes depended on the helical pitch and the relative birefringence.
Benqiao He et al. studied the random lasing in rhodamine 6G doped ethyl-cryanoethyl cellulose/acrylic acid cholesteric liquid crystal solution without scattering particles [47] and investigated the effects of the solution concentration and the thickness of the sample on the random lasing. The random lasing with coherent feedback was found to occur in an anisotropic solution of ethylcryanoethyl cellulose/acrylic acid, while only an amplified spontaneous emission was observed in an isotropic solution of ethylcryanoethyl cellulose/acrylic acid and acrylic acid solvent. The experimental results suggested that the cholesteric liquid crystal domains are also responsible for random lasing.
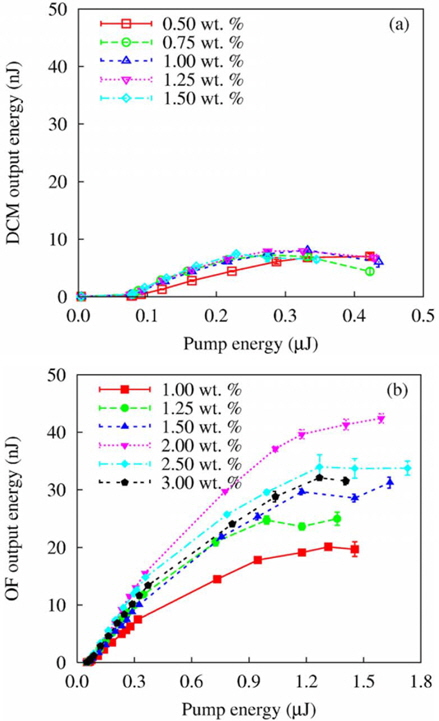
Ksenia Dolgaleva et al. proposed a highly efficient organic dye, oligofluorene, for lasing in cholesteric monomeric liquid crystal oligomers [48]. A comparative experimental study of the laser characteristics of monomeric cholesteric liquid crystals doped with oligofluorene and a dye, 4 (dicyanomethylene)-Z-methyl-6-(4-dimethylaminostryl)-4H-pyran (DCM), was performed.
Figure 7 shows laser output characteristics in the transverse single mode regime, in which the samples were placed at the focal point of the lens focusing the pump radiation.
The laser thresholds and the slope efficiencies of DCM and oligofluorene-doped cholesteric liquid crystals were similar in the transverse single-mode regime, but oligofluorene-doped cholesteric liquid crystals produced maximum output energy that was five times greater than that of DCM-doped cholesteric liquid crystals with superior temporal and spatial stability.
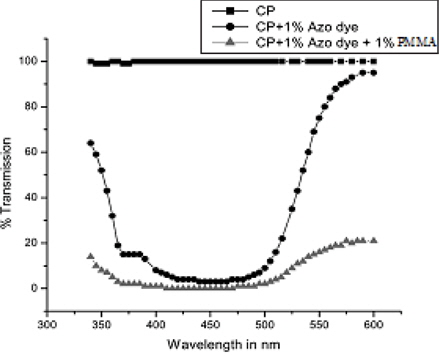
Vinita Dhulia et al. studied the mixture consisting of cholesteric liquid crystal, a polymer and the azo dye [49]. It was observed that poly methyl methacrylate (PMMA) could be used for stabilizing the cholesteric pelargonate (CP) and having controlled phase transitions by choosing the concentration of PMMA in the mixture of CP+PMMA. The phase transition temperature for pure CP as well as for the system CP+PMMA was found to decrease when azo dye was added in small percentage. Pure CP, which was transparent in the visible range, was nearly 100% absorptive in the range of 410 nm and 480 nm, and when azobenzene dye was added to pure CP and the CP+PMMA mixture.
Figure 8 shows that pure CP nearly has 100% transmission in the range of 440 nm and 600 nm. Doping CP with azo benzene dye resulted in nearly 0% transmission in the range of 410 nm and 480 nm. The intensity of transmission was found to reduce with the addition of PMMA.
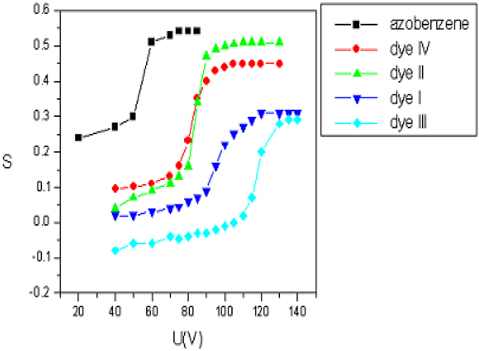
Carmina Plosceanu studied the optical dichroism of azodyes dissolved in a compensated mixture of cholesteryl chloride and cholesteryl-nonanoate under the action of an electric field [50]. The electric field produced significant changes oin the optical absorption of the dyes dissolved in the compensated cholesteric mixture. This was due to the changes of molecular arrangement under the electric field action. The order parameters of the dyes were determined by evaluating the optical dichroism of the dyes in these conditions. These results gave a measure of the electric field effect on the cholesteric-dye mixture.
Figure 9 shows the dependencies of the dyes’ order parameters (S) as a function of the electric tension applied to the cell. Three regions can be observed in the graph. In the first region, order parameters have small values corresponding to the small values of the electric tension. This presented a weak response to the electric field action. In the second region, for the tensions higher than a threshold value, the S parameters experienced a significant increase that corresponded to the cholesteric-nematic transition. In the third region, the order parameters had a slower increase and tended to reach a saturation value Smax.
M P.De Santo et al. investigated different solutions to improve stability in lasing emission from dye doped cholesteric liquid crystal mixtures [51]. It was found that suitable surface treatments, cell assembly and a translational or rotational movement of the cell with respect to the pumping beam could be useful in obtaining quite a stable intensity of laser emission in time. These results had been used to prepare a prototype of a laser system, able to emit several wavelengths in the visible range.
Takanishi et al fabricated novel hybrid structures composed of a dye-doped low-molecular-weight cholesteric liquid crystal sandwiched by multi-layered polymer cholesteric liquid crystal films on evaluating their lasing characteristics [52]. Lasing with an extremely reduced threshold (12 nJ/pulse) by a factor of 10 compared with that in a simple dye-doped low-molecularweight cholesteric liquid crystal cell was observed.
Guram Chilaya et al. considered two ways of using cholesteric liquid crystals in tunable dye lasers. These ways were cholesterics as distributed feedback medium and cholesterics as resonator mirrors [53]. In the dye doped distributed feedback cholesteric liquid crystal lasers, frequency tuning was achieved by exploiting light induced effects or by using a specially designed cell assembling a chiral dopant concentration gradient in combination with a suitable distribution of different dyes. Another approach represented the lasing in a multilayer system consisting of a dye doped isotropic solvent sandwiched between two cholesteric liquid crystal layers.
Laura Sutarlie et al. reported a study of using cholesteric liquid crystals doped with dodecylamine for detecting aldehyde vapors [54]. Cholesteric liquid crystals doped with dodecylamine changed color from red to yellow-green upon exposure to 300 ppmv pentyl aldehyde within 60 s. In contrast, no colorimetric response was observed when pure cholesteric liquid crystals were used. The cholesteric liquid crystals doped with dodecylamine showed good selectivity for pentyl aldehyde. This study demonstrated the potential applications of doped cholesteric liquid crystals as low-cost and portable gas sensors.
Shih-Hung Lin et al. investigated novel cone lasers, the tunabilities of their lasing feature and performance based on dyedoped cholesteric liquid crystal films with various liquid crystal birefringences (Δn). A unique conically symmetric lasing ring wi th a low energy threshold oc cur red at a specific nonzero oblique angle (θring) [55]. The lasing feature was tuned by varying birefringence. The dependence of lasing performance on birefringence for the three lasing signals was also measured, whose findings could be used to qualitatively identify positive interaction or competition among the three lasing signals.
L. Penninck et al. applied a plane wave model to simulate the spontaneous emission from a layer of cholesteric liquid crystal [56]. The spectral and angle dependence and the polarization of the emitted light as a function of the order parameter of the dye in the liquid crystal were stimulated. Measurements of the angle dependent emission spectra and polarization were found to be in good agreement with the simulations.
Shih C Y and Yeh HC studied the results of a photonic bandedge laser using dye-doped cholesteric liquid crystals combined with silver nanoparticles [57]. It was observed that when the silver nanoparticle surface, the plasmon resonance wavelength matched the excitation source wavelength, the fluorescence of dye molecules was increased by the large optical fields provided by surface Plasmon’s by enhancing the molecular excitation rate and a low lasing threshold and high pumping efficiency was achieved.
Longwu Li and Luogen Deng demonstrated a coherent random laser in a strongly scattering medium containing a dispersive solution of silver nanoparticles and laser dye 4-dicyanomethylene-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran in cholesteric liquid crystals that was injected into an oriented cell [58]. They experimentally verified the enhancement of the multiple scattering of silver nanoparticles and helical domains by the oriented cell confinement effect.
Xiangbao Yin investigated the relationship among the concentration of chiral reagent, the incidence angle of the pumping light, and the pitch of liquid-crystal display and made a tunable-wavelength laser and pumped the sample with a 532 nm laser output obtained from the Nd: YAG multi-frequency pulse laser [59]. It was found that as the incident angle of the pumping light shifted between 20° ~ 90°, the tuning range of the wavelength emitted by the laser reached 10.73 nm, ranging between 647.38 nm and 658.11 nm.
Mode competition of two-lasing modes at the photonic band edge from dye-doped cholesteric liquid crystal lasing was studied by Lin JH, Chen PY and Wu JJ by the alternation of temperatures [60]. The increase or decrease of the wavelengths from photonic band edges versus the alternation of temperature was attributed to the variation in the helical twist power. At a certain temperature, the intensity contrast and slope efficiency between long and short emission lasing peaks dominated from the gain or loss of the laser with regards to the position of the photonic band edge.
The review paper presents the work performed to improve the efficiencies of cholesteric liquid crystal lasers. Lasing with a low threshold was observed in a cholesteric liquid crystal doped with dyes. The order parameter of both the dye’s absorption and emission transition dipole moment was found to play an important role in determining the laser efficiencies. The lasing threshold of the cholesteric liquid laser was found to depend upon the dye concentration and sample thickness. Oligofluorene dye was found to be an excellent laser dye for use in cholesteric liquid crystal structures. Mirror-less lasing was observed in cholesteric liquid crystal doped with laser dyes. Recent advances to obtain tunable lasing from ultraviolet wavelengths through the visible range to infrared wavelength by employing cholesteric liquid crystal lasers have also been demonstrated. In the future, more effort will be made to improve the extracted output efficiency from a device viewpoint.