Tin oxide (SnO2) thin films are transparent and conductive, and it has potential applications in various technical areas such as resistors, gas sensors, transparent heating elements, antistatic coatings, etc [1-10]. It is well described in literature that electrical conductivity of films increases significantly without losing its optical transparency if a small amount of suitable [11-13] dopant is incorporated. On the contrary, addition of large amount of dopant diminishes the conductivity and crystallinity of the films [14,15] for a number of factors such as formation of mixed phases of host and dopant oxides, creation of different types of defect centers, generation of amorphous and crystalline states of the oxides, etc. Various film deposition techniques have been used to develop Sb doped SnO2, but the sol-gel processing exhibits good uniformity and better controlled composition [11,16,17] for thin film deposition. The purpose of the present work is three folds. The first purpose is to deposit Sn - Sb oxide films using sol-gel spinning technique, which is relatively less explored than sol-gel dipping but is very much suitable for coating circular substrates like glass disc and lens etc. Secondly, it is to study the effect of high dopant concentration(> 10 atom% Sb), especially on the optical properties like band gap as well as visible transparency, which may elucidate the selection of the dopant concentration for specific purposes. Hence, in the present work starting with SnCl4, 5H2O and SbCl3 precursor solutions, Sn - Sb oxide films belonging to MxM'yOz type, M = Sn and M' = Sb have been prepared and the atomic ratios of Sn : Sb were varied as 90 : 10, 70 : 30, 50 : 50, and 30 : 70. Moreover, the most common substrates for deposition of transparent conducting oxide films are various glasses that supply plenty of ions like Li+, Na+, Ca2+, Sr2+, Ba2+, etc. These ions can diffuse into transparent conducting oxides and may dope them either in the substitutional or interstitial site, depending on their size during the repeated annealing treatment of sol-gel processing. Unfortunately, the most common and most mobile ions in glasses are acceptors in the transparent conducting oxides and hence reduce the electrical conductivity. Deposition of a barrier layer on glass substrates prior to TCO film may inhibit such diffusion of ions. So, the third purpose of the present work was to investigate the effect of barrier layer by comparing electrical properties of films deposited on both bare and barrier layer coated soda lime silica (SLS) glass, which contains ~13% of sodium ion. For the measurement of optical band gap, coatings were also deposited on pure silica glass substrate to avoid the unwanted absorption of soda lime silica glass in the UV region.
2.1.1 Precursor for Sn - Sb oxide
Sols corresponding to 6 weight % equivalent SnO2 - Sb2O3 maintaining Sn : Sb atomic ratios as 90 : 10, 70 : 30, 50 : 50, and 30 : 70 were prepared. The required amount of hydrated stannic chloride (98% GR, Loba Chemie) was dissolved in a few ml of ethyl alcohol (for synthesis s. d. fine chem. Ltd.) and 1-propanol (for synthesis, E Merck India Ltd.) solvent mixture (1 : 1, by volume) and was stirred using a magnetic stirrer for 15 min. Similarly, required amount of Antimony (III) chloride (E Merck India Ltd., purity~ 98%) was dissolved and stirred separately for 15 min in the same solvent mixture. Next, the antimony chloride solution was added to the tin chloride solution slowly with simultaneous drop wise addition of conc. HCl, in order to increase the solubility of antimony chloride and to prevent the precipitation of antimony oxychloride. The solution was stirred for 1h and aged for 48 h for coating.
2.1.2 Precursor for silica
The 200 g precursor sol of 6 wt% equivalent SiO2 was prepared. The first step was to mix 52 ml tetraethoxyorthosilane with 101.5 ml 1-propanol. The solution was stirred for 10 min, and then, 0.86 ml of 1(N) HCl was added to the solution until acid : alkoxide mole ratio became 0.01 : 1. After 1 h of stirring, 8.4 ml of H2O was added drop wise to the solution. The water: alkoxide mole ratio was 2 : 1. Next, the solution was stirred for 140 min and mixed with 75.3 ml of 2 - butanol. The solution was stirred for another 30 min and aged for 24 h to obtain a wettable SiO2 sol.
2.2.1 Deposition of barrier silica layer
Ultrasonically cleaned Soda lime silica (SLS) glass substrate of 30 mm × 30 mm × 3 mm dimension was chosen for the coating operation. SiO2 layer was deposited on SLS substrate by the dipping technique (withdrawal speed, 16 cm/min). The coating was cured in air at ~ 450℃ for 0.5 h. Physical thickness of the barrier layer was 200 ± 25 nm.
2.2.2 Deposition of Sn - Sb oxide film
The films were deposited on three types of substrates (i) pure silica glass, (ii) bare SLS, and (iii) SiO2 coated (barrier layer) SLS glass by spinning,using the precursors of different Sn : Sb ratios. The concentration of Sn - Sb oxide precursor sol was 6 wt% equivalent SnO2 - Sb2O3 and the spinning rpm was 1,500. After each deposition, the coatings were cured in air at 500℃ for 0.5 h, and the whole process was repeated several times to increase the physical thickness of the coatings.
Transmission and absorption spectra of the coatings were recorded by UV-VIS-NIR spectrophotometer (Shimadzu UV3101 PC) for obtaining optical band gap of the films. Sheet resistance (Ω/☐) of the films was measured by two probe system. This was done by connecting the two probes of a multimeter (Philips PM2525) with two silver electrodes placed 1 cm apart on the coated surface. For deposition of silver electrodes, the condensed silver particles (Eltech Corporation, India) were dispersed into a suitable thinner to form a silver paste, and the paste was coated on the sample using a brush in the form of two narrow lines one cm apart. Next, the sample was heated at ~ 150℃ for ½ h for well adherence of the electrodes. A few of the samples were tested for evaluation of Hall mobility (μ), free electron carrier concentration (N) and resistivity (ρ) in a magnetic field of 0.51 T (Tesla) at room temperature by HEM 2000 (EGK Corporation, Korea), using four probe van der Pauw method. Thicknesses of Sn - Sb oxide films were measured elipsometrically (Gaertner Auto gain L116 B, Gaertner Scientific Corporation, USA) at 632.8 nm (He - Ne laser source).
The physical thickness of the Sn - Sb oxide films deposited on bare and barrier silica coated SLS glass substrates varied within a range from 965 ± 3 Å to 2,398 ± 3 Å. (Table 1). It is surprising that with the increasing Sb content, the physical thickness of the films decreased, although the concentration of precursor sol and coating application rate (4 consecutive depositions with 1500 rpm followed by curing after each deposition) were maintained at constant. This decrease was possibly due to decomposition of Sb2O5, which was formed within the film material with relatively high concentration for higher Sb content film. The decomposition usually occurs at ~ 380℃ with loss of oxygen. Another possible reason may be the presence of less hydrolysable SbCl3 in greater amount in the precursor sol of higher Sb content film, which reduces the target concentration (6 wt% equivalent SnO2 - Sb2O3) of the sol and in turn decreases the thickness of the film.

Substrate type, Sb atom %, physical thickness, percentage transmission, sheet resistance, and Hall measurement data of various Sn - Sb oxide films deposited on barrier layer coated and bare SLS substrate.
The Sn - Sb oxide films were deposited on pure silica glass for evaluation of optical band gap. To avoid the optical interference due to inhomogeneity of the films, only single deposition was made while measuring band gap. The absorption spectra do not show (Fig.1) any significant absorption band, but sharp absorption was observed below 350 nm. In the case of pure silica substrate, the tailing effect of the absorption (up to 500 nm in the visible) decreased its transmission (Fig. 2).
As no sharp absorption peak was observed, the optical density was utilized for evaluation of absorption co-efficient, α, of the films deposited on pure silica glass. The direct (Fig. 3 and 4) and indirect band gaps of the doped films were evaluated by following eqn.(1)[18].
Where α is the absorption coefficient, hv is the incident photon energy, A is a constant, Eg is the band gap of the material, and the exponent n depends on the type of transition; n = 1/2, 2, 3/2, and 3 correspond to allowed direct, allowed indirect, forbidden direct, and forbidden indirect transitions, respectively.
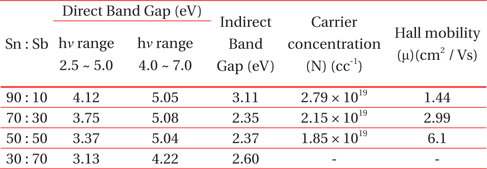
It is interesting to note that the direct band gap (Fig. 3; Table 2) becomes wider with decreasing Sb atomic ratio, which was clearly visualized for absorption at relatively low photon energy (2.5~5.0 eV). This follows the Moss - Burstein shift [19], which is caused by higher electron density in the conduction band. This is evident from the evaluated carrier concentration values that increased with decreasing Sb content (Table 2). The basic reason for the increase in carrier concentration with decreasing Sb content can also be explained with the presumption that all the dopant (Sb) has been utilized to substitute Sn atom, forming cassiterite Sb doped SnO2 material at a very low concentration of Sb (i.e. in our case Sn : Sb = 90 : 10). On further increasing Sb content, the disordered nature of SnO2 is created along with the formation of Sb2O5 phase. The mixed structure of the Sb2O5 phase and SnO2 phase accounts for the gradual decrease in the carrier concentration [15,20].
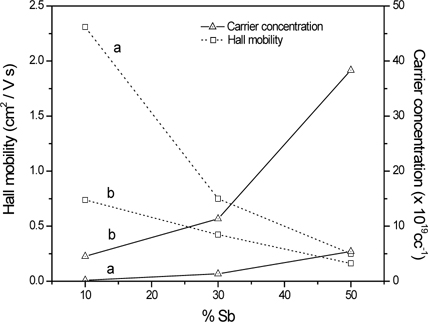
Band gap, carrier concentration, and Hall mobility of Sn - Sb oxide films deposited on pure silica substrates.
On the other hand, high band gap values were also obtained with respect to that of bulk material, if it was determined in the h
Sheet resistance and electrical resistivity of the Sn - Sb oxide films were measured by two probe method (Table 1). Minimum resistivity of ~2 × 10−2 Ω cm was obtained for the film deposited on barrier silica layer coated substrate using the precursor of Sn : Sb = 90 : 10.Sn - Sb oxide films deposited on bare SLS substrate showed higher sheet resistance values as well as higher resistivity than those deposited on barrier layer coated substrate, for all the compositions except Sn : Sb = 70 : 30 where no conductivity was observed irrespective of substrate nature. This was expected as it was mentioned earlier that Na+ ions diffuse into the film from SLS substrate during repeated annealing treatment. These ions act as an acceptor and lower the effective donor concentrations in Sn-Sb oxide film, which is a n-type semiconductor. Deposition of barrier layer may reduce this type of intrusion of acceptor ions and may cause betterment of electrical properties. For further confirmation of the above fact, electrical properties of the films deposited on bare and barrier silica layer coated SLS substrate were compared after evaluating Hall mobility (μ) and free electron carrier concentration (N), using four probe van der Pauw method for a few selected samples. The carrier concentration was in the order from 1018 to 1020 cc−1(Table 1). Hall mobility varied from 0.16 to 2.31 cm2 / Vs. Carrier concentration of the films on bare substrate was found to be relatively less with respect to that of the barrier layer coated substrate, clearly suggesting the possible presence of Na+ acceptor ion in the case of bare substrate.
Deposition of silica barrier layer thus could inhibit Na+ ion intrusion to some extent. On the other hand, the carrier concentration increased with increasing Sb content while Hall mobility decreased irrespective of the substrate nature (Fig. 5). The decrease in mobility is due to relatively high electron scattering of the films, due to increasing disorder in the crystalline phase with increasing Sb content. Minimum sheet resistance was obtained for 10 atom % Sb content film for both types of substrate, while bulk resistivity (= Sheet resistance x physical thickness) showed no such specific preferred composition because of different physical thickness of the films deposited on different substrates. No conductivity was observed for the films with maximum Sb content (Sn : Sb = 30 : 70) irrespective of substrate nature. The reason may be attributed to the development of Sb2O5 phase, which acts as an insulator [15, 20].
Sn - Sb oxide films with wide variation of Sb (10.0, 30.0, 50.0, 70.0 atom%) were deposited on glass (bare and barrier layer coated) by sol-gel spinning technique and characterized by Hall mobility, carrier concentration, and transmission in the visible, optical band gap studies. In this study, blue shift of band gap was observed with respect to bulk band gap. Moss-Burstein shift in band gap was consistent with the carrier concentration of the films. Physical thickness of the films decreased with increase in % Sb, which was possibly due to decomposition (380℃) of Sb2O5. Sheet resistance and resistivity of the films deposited on barrier layer coated substrate was found to be relatively less, which implies the inhibition to intrusion of Na+ ion by barrier layer during curing.