Lung cancer ranks first as a cause of mortality and is the second most common cancers [1]. The relationship between cancer cells and the surrounding extracellular matrix (ECM) deserves special attention because a key step in tumor development involves the invasion of malignant cells into their immediate environment [2]. The components of the ECM are a major hindrance to the proliferation, migration and maturation of endothelial and tumor cell. To overcome this interstitial tissue barrier, tumor cells express themselves as a complex array of specific proteases to degrade the ECM [3]. The continuous processes of ECM turnover accelerated by tumor derived proteases, along with the abundance of remodeled ECM, pave the way for neovascularization. The macromolecules of the ECM also play a major role in microvascular morphogenesis. Vessel destabilization, which includes the weakening of intracellular contacts between endothelial cells and the local proteolysis of matrix proteins, is necessary to initiate angiogenesis. Endothelial cells during angiogenesis extensively remodel the ECM around them by digesting the pre-existing basal membrane and re-synthesizing collagen, proteoglycan (PG) and glycosaminoglycans (GAGs), ultimately forming the ECM of new capillaries [4]. The role of ECM in the tumor’s microenvironment is not limited to being a barrier against tumor invasion. The ECM is a reservoir of cell binding proteins and growth factors that affect tumor cell behavior.
Metastasis of cancer cells involves multiple processes and various cytophysiological changes, including changes in the adhesion capability between cells and the ECM and interference with intercellular interactions. Thus, any degradation of the ECM and the components of the basement membrane caused by the concerted actions of proteinases, such as serine proteinase, matrix metalloproteases (MMPs), cathepsins, and plasminogen activator (PA), plays a critical role in tumor invasion and metastasis [5]. Among these enzymes, MMP-2 and MMP-9 are able to degrade most components of the ECM directly and are deeply involved in cancer invasion and metastasis [6]. Therefore, any inhibition of the migration or the invasion mediated by MMP-2, and MMP-9 may be a key feature for the prevention of cancer metastasis [7].
Many epidemiological and animal studies have shown that diet rich in fruits and vegetables decreased the occurrence of cancers [8, 9]. Capsaicin (CAP, 8-methyl-N-vannilyl- 6-nonenamide) is the chief pungent principle found in the hot red peppers and the chili peppers of the plant genus Capsicum and has long been used in spices, food additives and drugs [10]. Different studies have revealed the divergent functions of CAP, including its anti-carcinogenic and anti-angiogenic effect [11-15]. Our previous studies have also substantiated the anti-cancer activity of CAP in several dimensions [16-18].
Hence the current investigation aimed at assessing the effect of CAP in regulating extracellular matrix components and proteases during benzo(a)pyrene induced experimental lung carcinogenesis.
Benzo(a)pyrene and CAP were purchased from Sigma Chemicals, St. Louis, U.S.A. All other chemicals were of analytical grade and were procured from SRL Chemicals Pvt. Ltd., Mumbai, India.
Healthy male Swiss albino mice weighing 20 ─ 25 g and (8 ─ 10 weeks old) were obtained from the Veterinary College, Chennai, and were used in this experiment. This study was ethically approved by the Ministry of Social Justices and Empowerment, Government of India and by the Animal Ethics Committee of our Institution (IAEC No. 01/024/08). The animals were housed in an environment with a controlled temperature (26 ± 2℃) and a 12 hours day/night cycle. They were fed a standard rat/mice pellet diet (Hindustan Lever Ltd., Mumbai) under the trade name Amrut rat/mice feed and were given access to water
Experimental animals were divided into four groups of six mice each as follows:
Group I: Included the control animals that received olive oil throughout the course of the experiment.
Group II: Included animals that were treated with benzo( a)pyrene (50 mg/kg body weight (bwt) dissolved in olive oil) orally twice a week for four successive weeks.
Group III: Animals received CAP alone (10 mg/kg bwt dissolved in olive oil) intraperitoneally once a week for 14 weeks to assess the cytotoxicity (if any) induced by CAP.
Group IV: Animals received benzo(a)pyrene (as in group II) along with CAP (10 mg/kg bwt dissolved in olive oil) intraperitoneally.
CAP treatment was started one week prior to the first dose of benzo(a)pyrene and was continued for 14 weeks. At the end of the experimental period (14th week), animals were sacrificed by cervical decapitation under ether anesthesia and their lungs were excised immediately and washed with ice cold saline. A 10% homogenate of the washed tissue (lung) was prepared in 0.01 M phosphate buffer (pH 7.4). The homogenate was centrifuged at a speed of 12,000 × g for 30 minutes in a refrigerated high speed centrifuge at 4℃. Blood was also collected, and the serum was separated for other analyses. The biochemical analyses that were carried out in the supernatant and in the serum are described in the next section.
Assay of ECM components: The hydroxy proline content was determined [19]. The elastin residue that remained insoluble after cyanogen bromide (CNBr) and formic acid digestion was determined by using a ninhydrin assay [20]. PGs were measured in terms of hexosamine [21] and uronic acid [22], and GAGs were extracted [23] and analyzed [24].
Lung histopathology (Masson’s trichome staining): Lung tissue samples were histologically evaluated. Portions of specimens were fixed in 10% formalin and embedded in paraffin wax. Sections were then to a thickness of 4 μm and stained for collagen by using Masson’s trichome method.
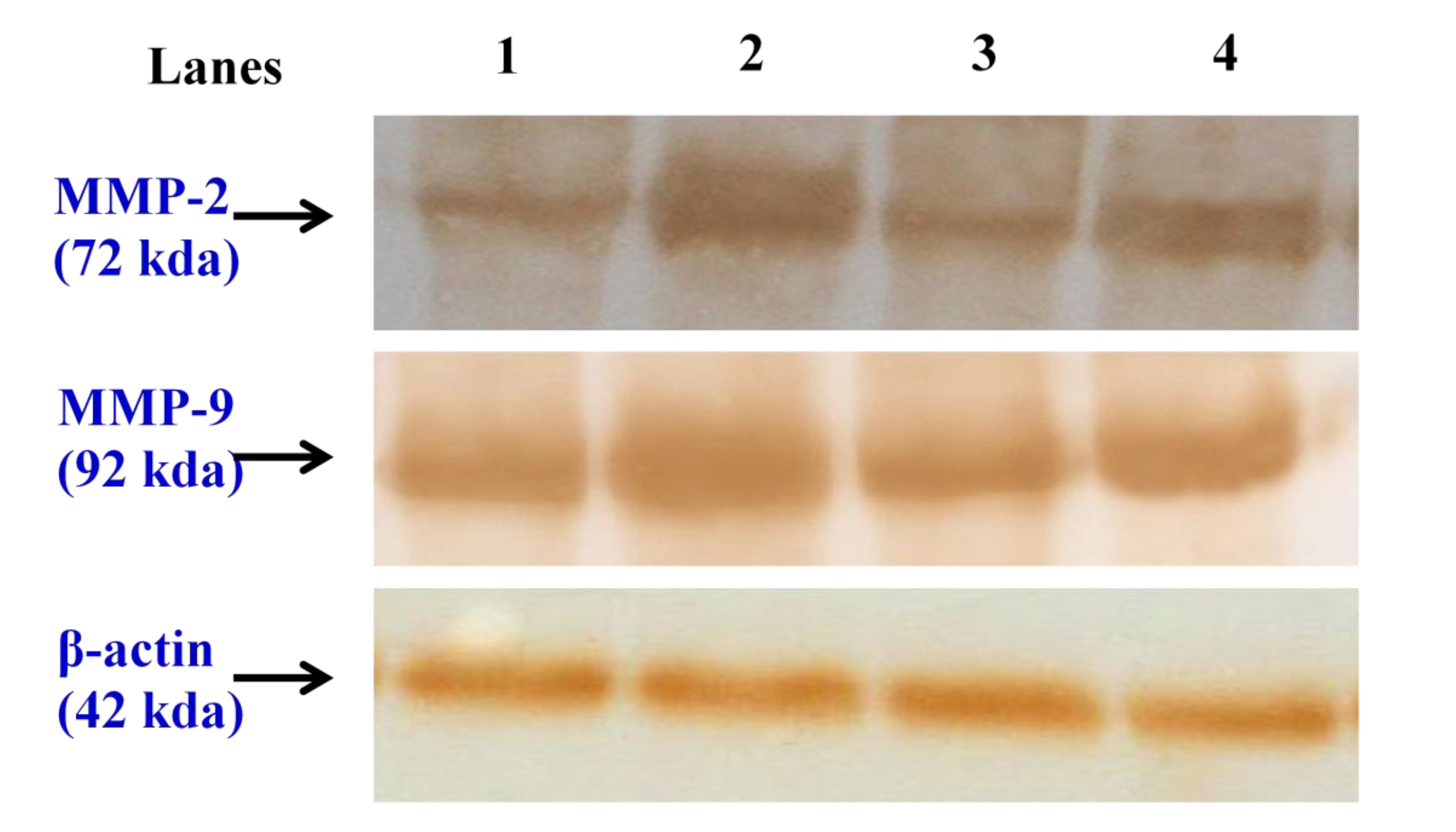
Western blot analyses: Approximately 50 μg of the total cell lysate was mixed with an equal volume of 2 × sample buffer, boiled for 5 minutes at 95oC, cooled, loaded on each lane of a 10% polyacrylamide gel, and separated by using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) at room temperature. The resolved proteins were electrophoretically transferred to nitrocellulose membranes. The membranes were then blocked in 5% non fat milk in Tris-buffered saline (TBS) with 0.1% Tween 20 for 1 hour at room temperature, and probed with the following primary antibodies: MMP-2 and MMP-9 rabbit polyclonal antibodies at a concentration of 3 μg/mL and beta actin (Sigma) mouse monoclonal antibody at a dilution of 1 : 2000 overnight at 4oC. The blots were then extensively washed with TBS with 0.1% Tween 20 and then incubated with anti rabbit and anti-mouse horseradish peroxidase (HRP) labeled secondary antibody (Genei, Bangalore, India) at a dilution of 1 : 2000 for 1 hour at room temperature. After extensive washes in Tris-buffered saline- Tween (TBS-T), the bands were visualized by treating the membranes with 3,3’-diaminobenzidine tetrahydrochloride (SRL, Mumbai, India). The membranes were photographed and quantitatively analyzed by using image analysis software (image j, NIH, U.S.A.). The densitometry data are presented in bar graphs and represent the “fold change” as compared with the controls.
Data analysis: All data were expressed as mean ± standard deviation (S.D.) for six mice. The results were analyzed statistically (SPSS Software Package) by using the one-way analysis of variance (ANOVA). Post-hoc testing was performed for inter-comparisons by using the least significant difference (LSD).
Fig. 1 shows the levels of collagen measured in terms of hydroxy proline in the lungs of the control and the experimental groups of animals. A significant (
Fig. 2 shows the results from the histopathological examinations (Masson’s trichome staining) of the lung section of the control and the experimental animals. Group I control animals revealed normal architecture (Fig. 2a).
Lung cancer bearing animals (group II) showed hyper proliferative cells with increased collagen deposition, (Fig. 2b). Group III animals exhibited normal architecture indicating the non toxic nature of CAP (Fig. 2c). Pretreatment with CAP markedly reduced collagen accumulation in Group IV animals, thereby preserving the near normal architecture (Fig. 2d).
Table 1 shows the levels of extracellular matrix components in the control and the experimental groups of animals. The levels of ECM components, namely, elastin, uronic acid and hexosamine, were found to be markedly (
Fig. 3 depicts the levels of glycosaminoglycans in the control and the experimental groups of animals. Significant (
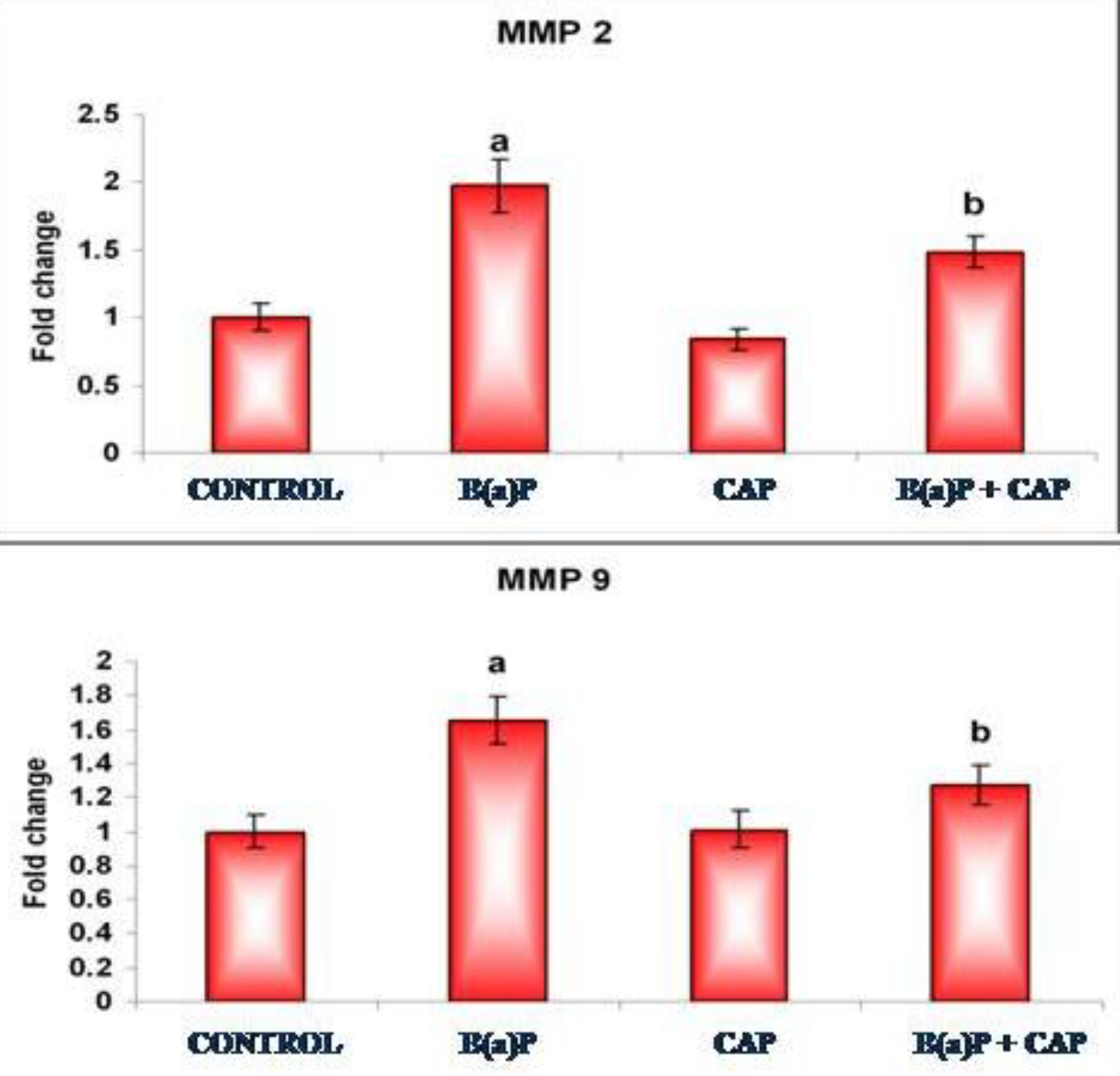
Fig. 4a,4b, depicts the immunoblotting and the densitometric analyses of MMP-2 and MMP-9, respectively, in the control and in the experimental groups of animals. The protein expressions of MMP-2 and MMP-9 was profoundly increased in the tumor bearing animals (group II) when compared with the normal control animals of group I. Animals in the CAP treated groups showed markedly reduced protein expressions of MMP-2 and MMP-9 when compared with the lung cancer bearing group II animals.
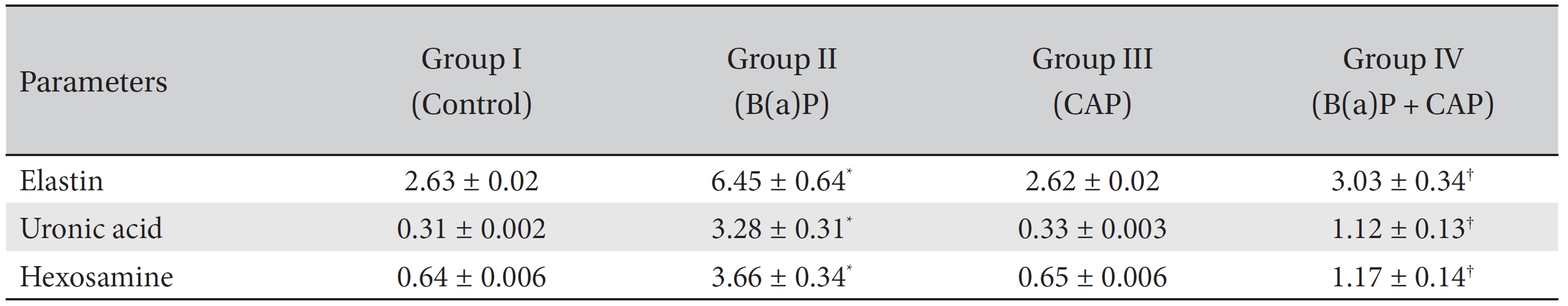
Levels of extracellular matrix components in the lungs of the control and the experimental group of animals
Until recently, the alveolar ECM was considered only to be a static structural component of lung tissue. However, the ECM is now known to serve as a modulator of cell growth and development, inflammation, angiogenesis, cell migration, tissue differentiation, and tissue repair [25]. As these processes occur, they influence the cellular and matrix composition, and the ultimate fate and function of newly formed tissue. Collagen is the predominant extracellular matrix protein, and tumor tissue has a higher collagen content than in normal tissue [26]. The increase in collagen synthesis may perhaps be due to the stimulation of local dormant fibroblasts by various stimuli, such as reduced oxygen tension and increased lactate production or released tissue amines [27]. The collagen production may be vital in establishing basal membrane integrity and may provide a scaffold for new vessel ingrowths and inflammatory cell migration. As the inflammatory cells are attracted and guided into the wound by the newly synthesized collagen lattice, the tumor associated macrophages, in turn, release chemotactic factors to stimulate migration of fibroblasts for additional collagen synthesis. Collagen may also serve as a reservoir for growth factor and/or modulate their activities thereby setting the stage for accelerated growth [28]. Elastin is a resilient connective tissue protein, and it accounts for 2% to 4% of the ECM. Elastin provides elasticity, in addition to the tensile strength conferred by collagen fibers. Studies using cultured skin fibroblasts have demonstrated that tobacco smoke components including benzo(a)pyrene, induce an increase in collagen and the elastin components of the ECM, which, in turn cause premature skin aging during cancerous condition [29]. An increase in the biosynthesis of collagen measured by the formation of hydroxyproline during pulmonary damage has been reported [30]. The results in that report are in line with our present findings as we observed increase in collagen and elastin contents in lung cancer bearing animals. CAP treatment significantly decreased collagen and elastin levels, which clearly indicates that CAP influences the entire process of collagen and elastin metabolism.
The role of GAGs during ECM remodeling is transitory: they provide a highly hydrated, gel like provisional matrix following fibrin degradation, only to be subsequently replaced by collagen. The relative amounts of the various GAGs present in tumor tissue are found to be higher than those found in normal tissues [31]. Hyaluronate (HA), chondroitin sulfates and dermatan sulfates are the major GAGs present. Of these three GAG fractions, a relative abundance of HA is found in tumor tissue [32]. Tumor tissue is strongly positive for HA, and an accumulation of HA is immune localized at the tumor’s margins [33]. As HA is deposited, it causes a modification in the hydration and the permeability of the fibrillar network and in the subsequent phases, and it is reduced to low molecular weight fragments, which stimulates angiogenesis [34]. HA is known to complex with collagen, and this has led to the proposal that HA collagen interactions may affect collagen fibrillogenesis [35]. Experimental evidence shows that chondroitin sulfate levels are also markedly increased during cancerous conditions [36], and that corroborates our present findings. Dermatan sulfate containing PGs are known to interact with collagen and to contribute to the organization and the strength of the fibrillar network. The low levels of GAGs in the CAP supplemented animals could terminate the enhanced levels of chemotactic and mitogenic stimuli for various cells, thereby removing the environment that accelerates tumor progression.
MMPs are a family of highly conserved endopeptidases that are capable of degrading the ECM. Over 25 well characterized members of this proteinase family have been identified. They play a key role not only in the normal processes of ECM degradation, but also in pathological processes, including tissue remodeling of inflammatory disease, cancer invasion, and metastasis [37]. Much evidence indicates that MMPs are actively involved in the metastatic process and a positive correlation between tumor progression and the expression of multiple MMP family members (MMP-1, MMP-2, MMP-7, MMP-9, MMP-11 and Membrane-type 1 (MT1)-MMP) in tumor tissues has been demonstrated in numerous studies [38-41]. Furthermore, experimental evidences have shown elevated activities of MMP-2 and 9 during lung cancer, which is consistent with our present findings [6, 42]. Numerous studies have suggested that pharmacological targeting of MMP activity might provide a mechanism to prevent cancer dissemination [5]. Therefore, inhibition of the invasion mediated by MMP-2 and 9 may be a key feature for the prevention of cancer metastasis. In our present study, CAP treatment effectively down regulated MMP-2 and 9 levels, thus revealing its potent effect on tumor metastasis.
Our present investigation clearly indicates that CAP is effective in regulating the components of extracellular matrix and the proteases, which in turn paves the way for its anti-cancer action against experimentally induced lung cancer.