Rheum undulatum L. has traditionally been used for the treatment of many diseases in Asia. However, its anti-proliferative activity in cancer has still not been studied. In the present study, we investigated the anti-cancer effects of methanol extract of Rheum undulatum L. (MERL) on human adenocarcinoma gastric cell lines (AGS).
To investigate the anti-cancer effect of MERL on AGS cells, we treated the AGS cells with varying con¬centrations of MERL and performed 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays. Cell cycle analyses, measurements of the mitochondrial membrane potential (MMP), caspase activity assays and Western blots were conducted to determine whether AGS cell death occurred by apoptosis.
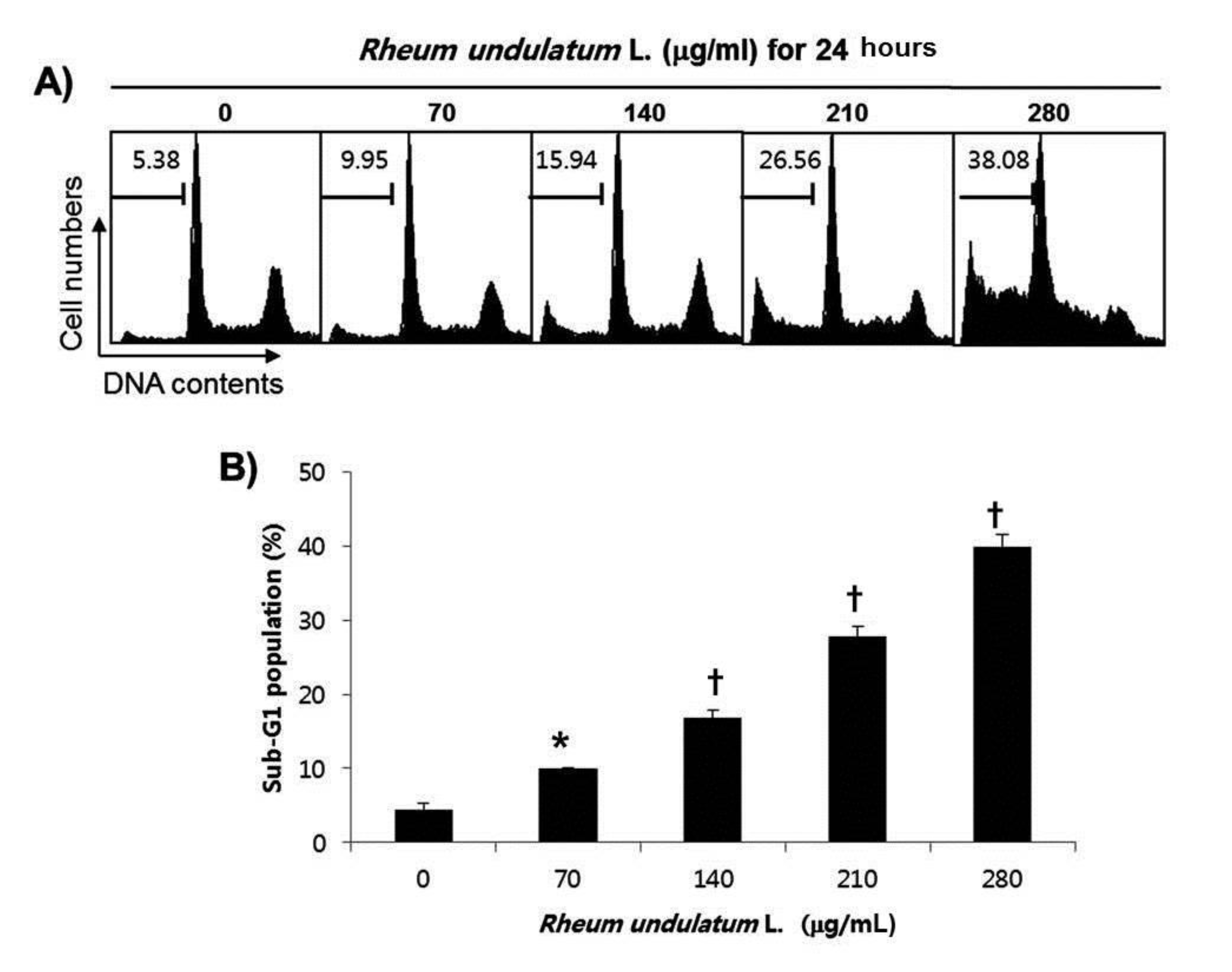
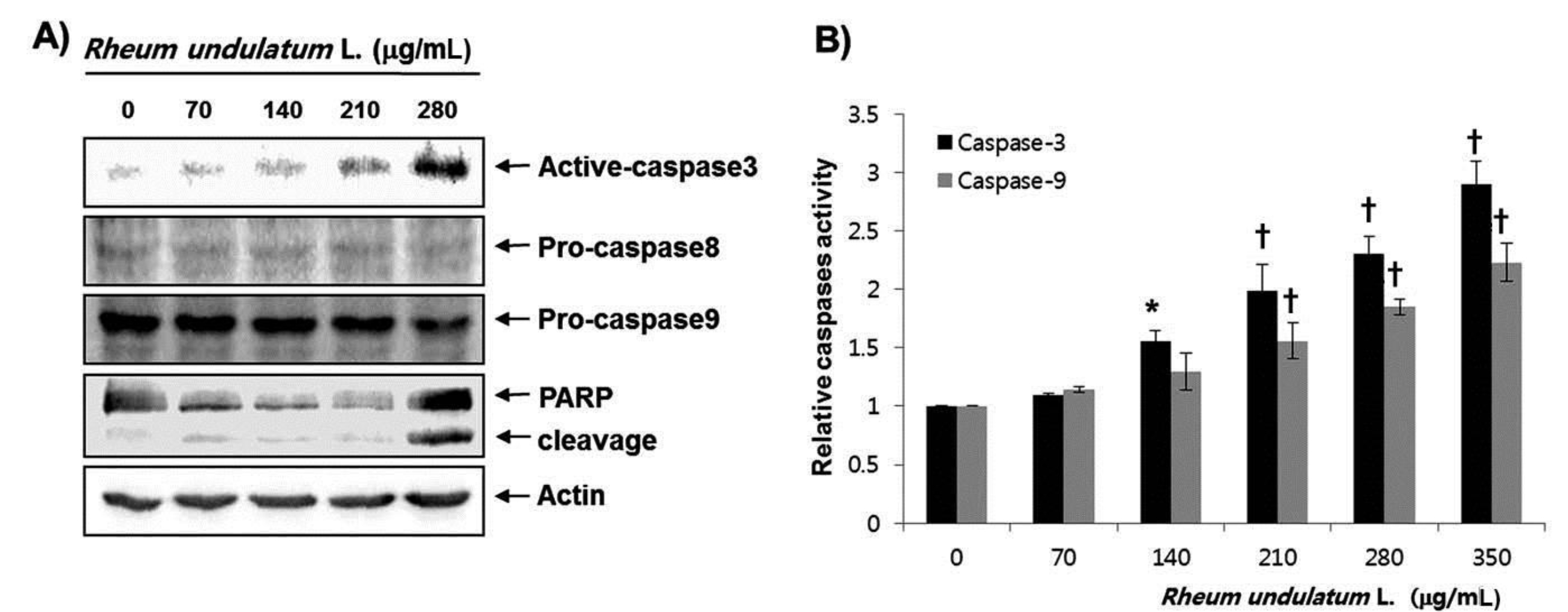
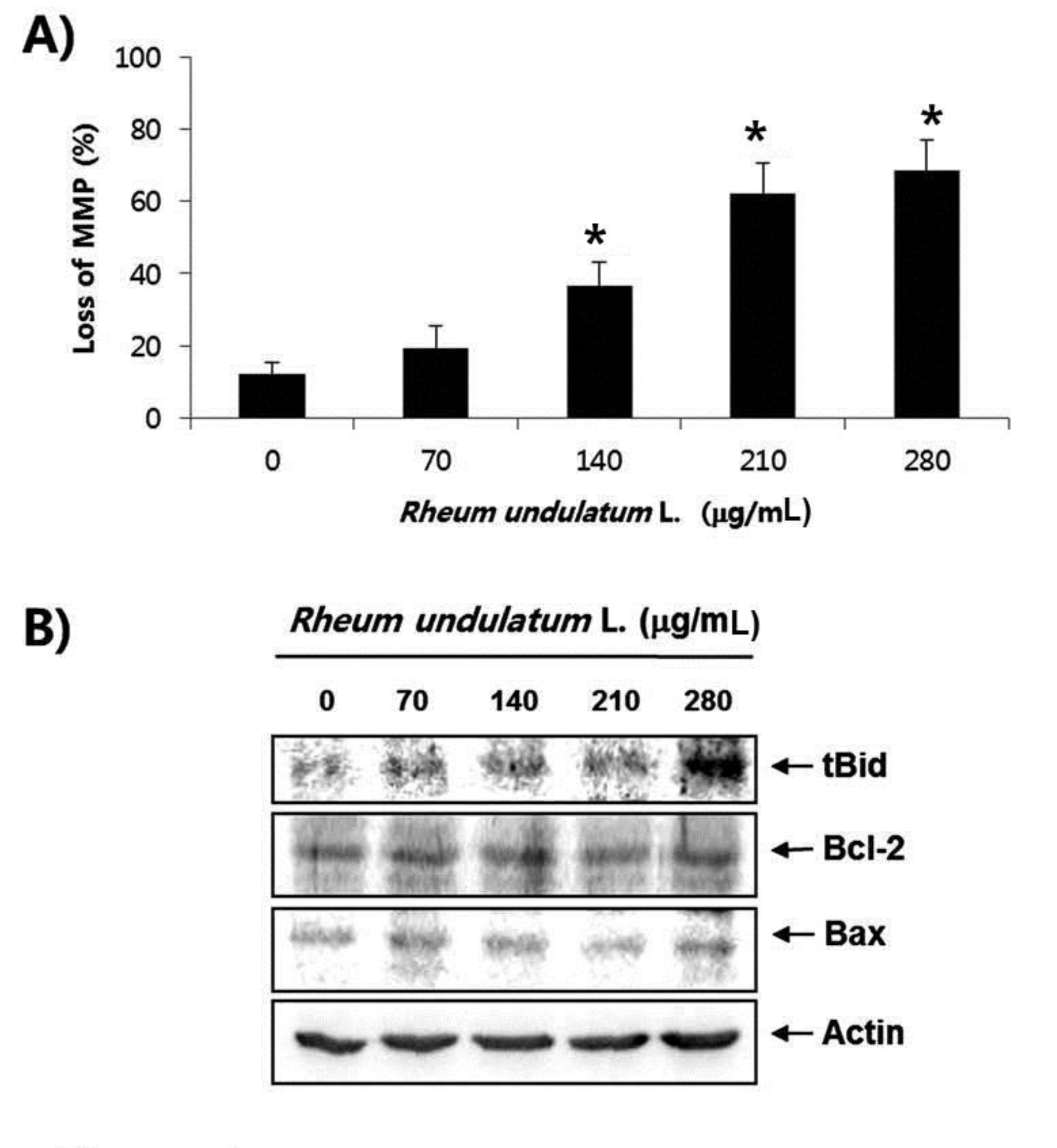
Treatment with MERL significantly inhibited growth of AGS cells in a concentration dependent manner. MERL treatment in AGS cells leaded to increased accumulation of apoptotic sub G1 phase cells in a concentration dependent manner. In control cultures, 5.38% of the cells were in the sub G1 phase. In MERL treated cells, however, this percentage was significantly increased (9.95% at 70 μg/mL, 15.94% at 140 μg/mL, 26.56% at 210 μg/mL and 38.08% at 280 μg/mL). MERL treatment induced the decreased expression of pro-caspase-8 and -9 in a concentration dependent manner, whereas the expression of the active form of caspase-3 was increased. A subsequent Western blot analysis revealed increased cleaved levels of poly (ADP-ribose) polymerase (PARP) protein. Also, treatment with MERL increased the activities of caspase-3 and -9 compared with the control. MERL treatment increased the levels of the pro-apoptotic truncated Bid (tBid) and Bcl2 Antagonist X (Bax) proteins and decreased the levels of the anti-apoptotic B-cell lymphoma 2 (Bcl-2) protein, whose is the stabilization of mitochondria. However, inhibitions of p38, extracellular signal regulated kinases (ERKs) and C-Jun N-terminal kinases (JNK) by MERL treatment did not affect cell death.
These results suggest that MERL mediated cell death is associated with an intrinsic apoptotic pathway in AGS cells.
Recently, many researchers have been looking for better anti-cancer agents from traditional medicinal herb [1]. Among cancers, gastric cancer in men is the fourth most frequently diagnosed cancer and the third most frequent cause of cancer death worldwide [2, 3]. Although cancer treatment including surgery, radiation therapy and chemotherapy has advanced in recent years, these therapies have many limitations because of low response and poor survival [4]. Therefore, the development of more effective strategies to improve the survival rate for gastric cancer patients is important.
In general, apoptosis has two major pathways: the death receptor pathway (extrinsic) and the mitochondria dependent pathway (intrinsic). The interaction between apoptosis inducing ligands and death receptors initiates an extrinsic pathway at the cell’s membrane and subsequently activates caspase-8 by the formation of a death induced signaling complex (DISC). The activated caspase-8 directly activates effecter caspases such as caspase-3, or cleaves BH3 interacting domain death agonist (Bid) to truncated Bid (tBid), leading to intrinsic pathway activation via mitochondrial dysfunction. As a result, cytochrome
The 3-(4, 5-dimetylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), propidium iodide (PI) and 5,5′, 6,6′-tetrachloro- 1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC- 1) used in this study were purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS) and caspase activity assay kits were obtained from GIBCO-BRL (Gaithersburg, MD) and R&D Systems (Minneapolis, MN), respectively. The extracellular signal-regulated kinase (ERK)-specific inhibitor, PD98059, the c-Jun N-terminal Kinase (JNK)-specific inhibitor, SP600125, and the p38 mitogen activated protein kinase (MAPK) specific inhibitor, SB203580 were purchased from Calbiochem (San Diego, CA). Enhanced chemiluminescence (ECL) kits were purchased from Amersham (Arlington Heights, IL). All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
For the preparation of MERL, the powder of a methanol extract of
The AGS human gastric adenocarcinoma cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained at 37°C in air at 95% humidity with 5% CO2 in RPMI1640 supplemented with 10% heat inactivated FBS, 2 mM glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
To investigate cell viability, we seeded the cells in 6 well plates at a density of 2 × 105 cells per well and stabilized them for 24 hours. The cells were then treated with various dose of MERL for the desired times. The MTT working solution (0.5 mg/mL) was then added to the culture plates, and the cells were incubated continuously at 37°C for 2 hours. The culture supernatant was completely removed from the wells, and DMSO was added to completely dissolve the formazan crystals. The absorbance of each well was measured at a wave length of 540 nm by using a microplate reader (Molecular Devices, Palo Alto, CA). The effect of MERL on the inhibition of cell growth was assessed on the basis of the percentage of cell viability, where the vehicle treated cells were considered 100% viable.
Following treatment with MERL, cells were trypsinized, washed with phosphate buffered saline (PBS), fixed in 75% ethanol, and stored at 4˚C overnight. Prior to analysis, cells were again washed with PBS, suspended in a cold PI (Sigma) solution, and incubated at room temperature in the dark for 30 minutes. A fluorescence activated cell sorting (FACS) can flow cytometry system (Becton-Dickinson, San Jose, CA) was used for the performance of flow cytometry analyses.
Cells were harvested and washed twice in PBS at 4°C. Total cells lysates were lysed in lysis buffer. The supernatants were collected and the protein concentrations were then measured by using protein assay reagents (Bio-Rad Laboratories, Hercules, CA). For Western blotting, equal amounts of protein extracts were denatured by boiling at 95°C for 3 minutes in sample buffer (0.5 M Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate (SDS), 20% glycerol, 0.1% bromophenol blue, 10%
The activities of the caspases were determined by using colorimetric assay kits, which utilize synthetic tetrapeptides (Asp-Glu-Val-Asp DEVD for caspase-3, and Leu-Glu- His-Asp (LEHD) for caspase-9) labeled with p-nitroaniline (p-NA). Briefly, MERL treated and untreated cells were lysed in the supplied lysis buffer. The supernatants were collected and incubated with the supplied reaction buffer containing dithiothreitol (DTT) and DEVD-pNA or LEHD- pNA as substrates at 37°C. The reactions were measured by using a microplate reader to record the changes in the absorbance at a wavelength of 405 nm.
Mitochondrial membrane potential (MMP) was determined by using the dual emission potential sensitive probe JC-1. The cells were collected and incubated with 10 μM JC-1 for 20 minutes at 37°C in the dark. The cells were then washed once with PBS and analyzed by using a flow cytometer [14].
Unless otherwise indicated, each result is expressed as the mean ± standard deviation (S.D.) of data obtained from triplicate experiments. A statistical analysis was performed by using a paired Student’s
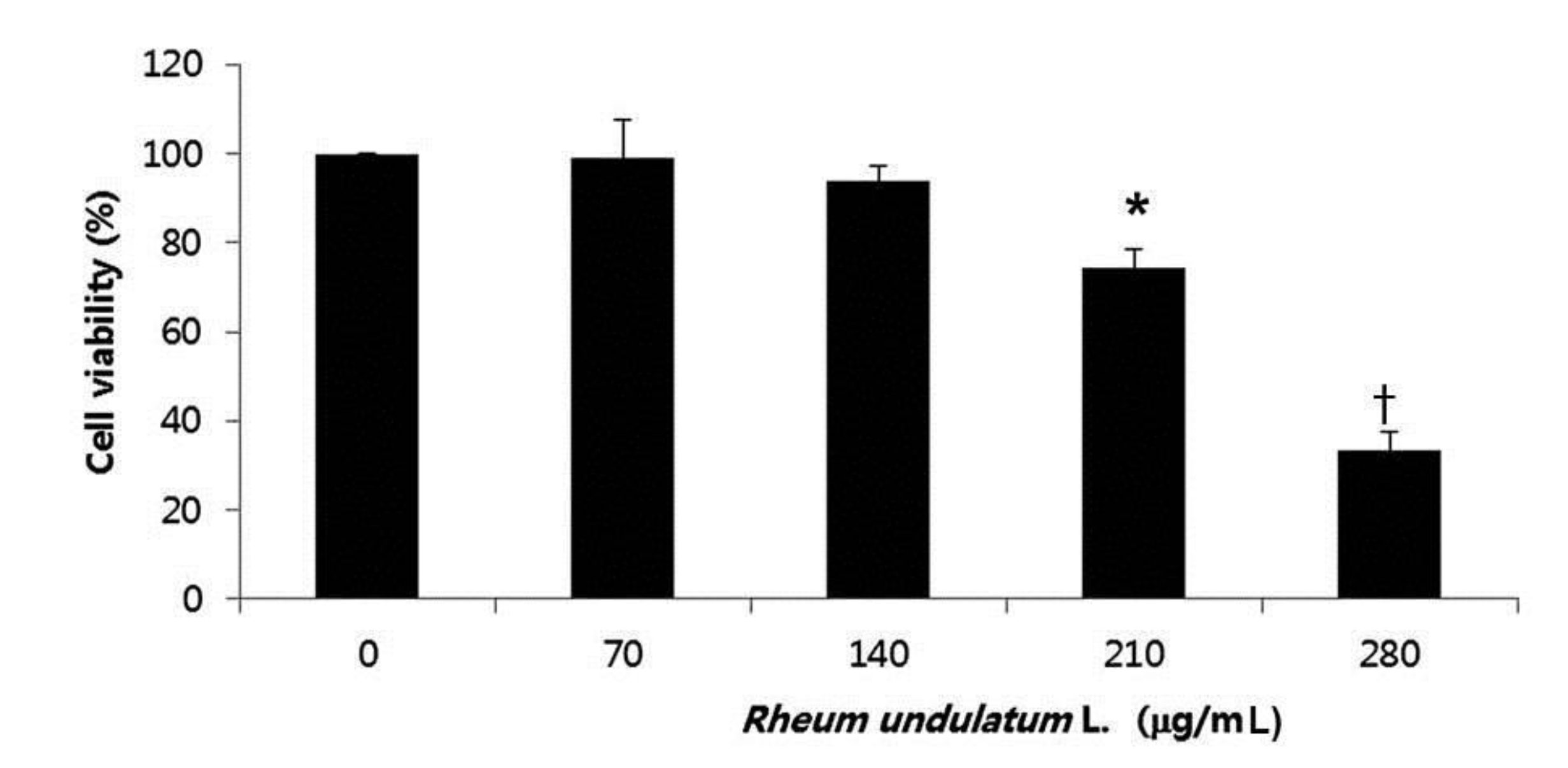
To determine the effect of MERL on cell growth in AGS cells, we treated the cells various concentrations of MERL for 24 hours; then, we used an MTT assay to measure the cell’s viability. As shown in Fig. 1, the cell’s viability was significantly decreased as a result of the MERL treatment in a concentration dependent manner. These results suggested that exposure of the AGS cells to MERL decreased cellular viability in a concentration dependent manner (Fig. 1).
We next performed a flow cytometric analysis to detect apoptotic dead cells to determine whether MERL induced cell death resulted from apoptosis. As indicated in Fig. 2, MERL treatment of AGS cells leaded to increased accumulation of apoptotic sub G1 phase cells in a concentration dependent manner. In the control cultures, 5.38% of cells were in the sub G1 phase. In MERL treated cells, however, this percentage was significantly increased (9.95% at 70 μg/mL, 15.94% at 140 μg/mL, 26.56% at 210 μg/mL and 38.08% at 280 μg/mL). These results suggest that MERL induced growth inhibition was related to the induction of apoptosis in AGS cells.
Caspases are known to be important mediators of apoptosis in both the intrinsic and the extrinsic pathways. This executionary phase induces activation of cytoplasmic endonuclease, which degrades nuclear material, activation of proteases, which degrades cytoskeletal proteins and nuclei, and cleavage of various substrates including polymerase (PARP), which serves as an apoptosis marker [15]. As shown in Fig. 3a, MERL treatment induced a decrease in the expression of pro-caspase-8 and -9 in a concentration dependent manner, whereas the active form of caspase-3 was increased. Subsequent Western blot analyses revealed increased cleaved levels of the PARP protein. We next attempted to quantify the proteolytic activation of caspases due to the MERL treatment. As shown in Fig. 3b, treatment with MERL increased the activities of caspase-3 and -9 compared with the levels for the controls. These results indicate that MERL treatment induces apoptosis via activation of caspases in AGS cells.
Mitochondria, which are specialized organelles, play a critical role in apoptosis. Intermembrane spaces of mitochondria contain many pro-apoptotic proteins including cytochrome
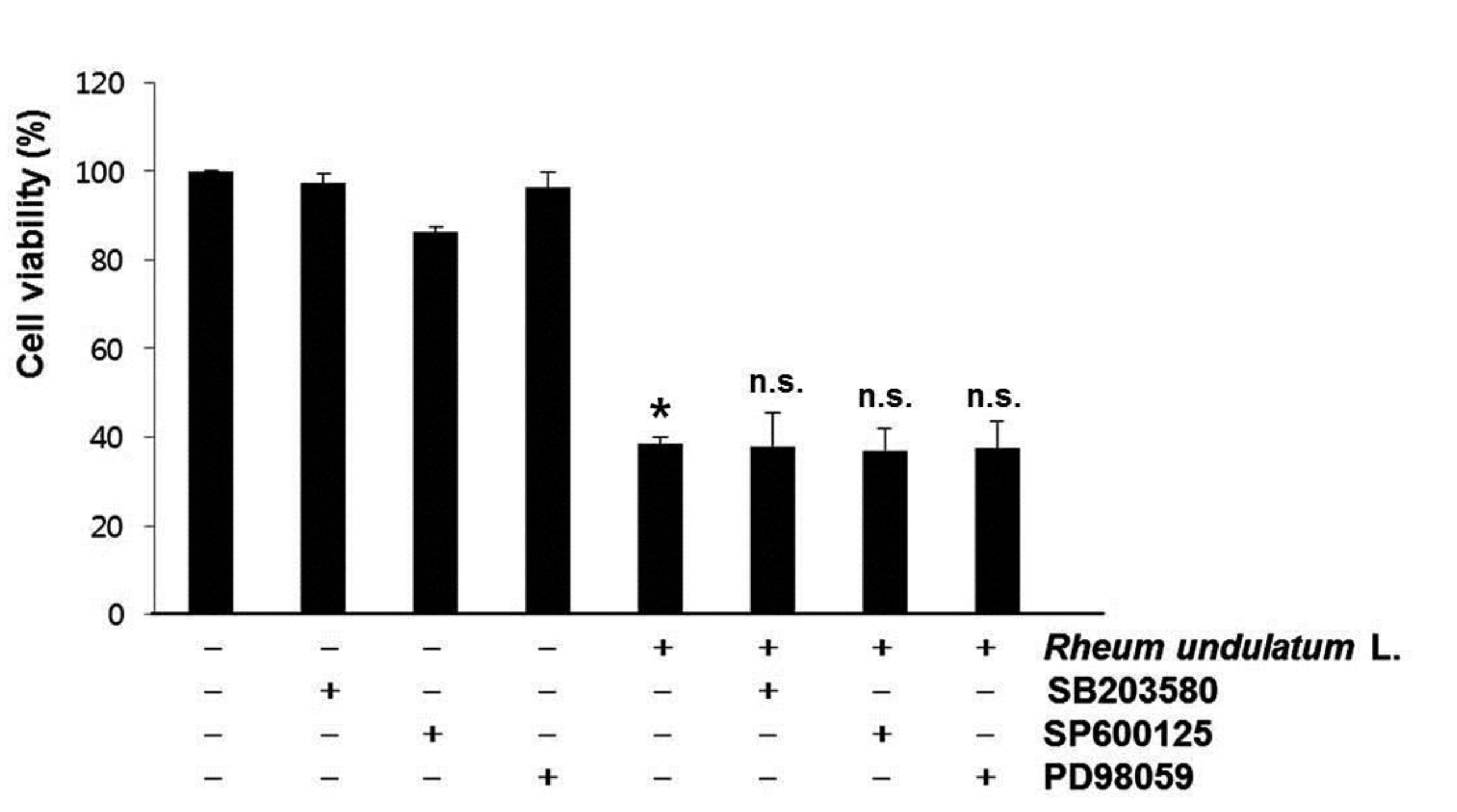
The MAPK signaling pathway functions as a critical regulator of cell survival and proliferation [20]. The MAPKs, including p38 MAPK, ERKs and JNKs also play fundamental roles in survival, proliferation, and apoptosis [21]. To determine whether these signaling pathways played a role in the MERL induced apoptotic response, we pretreated the cells with a specific inhibitor of MAPK and then measured the cell’s viability by using the MTT assay. As shown in (Fig. 5), pretreatment with SB203580 (a specific inhibitor of p38 MAPK), SP600125 (a potent inhibitor of JNK), or PD98059 (a potent inhibitor of ERK) did not have an effect on the response to MERL treatment. Taken together, these results suggest that MERL induced cell death is associated with the intrinsic apoptotic pathway in AGS cells.
In this study, we used a methanol extract of
Apoptosis can be initiated by two pathways, the extrinsic and the intrinsic pathways. The extrinsic apoptotic pathway can be triggered by interactions between transmembrane death receptors and their cognate ligands. These interactions result in recruitment of the associated death domain (FADD) and caspase-8 to the DISC, leading to activation of caspase-8 [15, 26]. The intrinsic apoptotic pathway involve non receptor mediated stimuli that produce mitochondrial mediated signals, resulting in an opening of mitochondrial permeability transition (MPT) pore, loss of the mitochondrial membrane potential and release of pro-apoptotic proteins such as cytochrome
Our study showed that the MERL reduces cell proliferation and induced apoptosis, which was confirmed by an increased accumulation of the sub G1 phase. Furthermore, MERL induced apoptosis was shown to be associated with activation of caspases, and mitochondrial dysfunction based on the decreased Bcl-2 and the increased tBid and Bax proteins levels. In addition, specific inhibitors of MAPK had no effects on MERL induced apoptosis in AGS cells. These findings suggest that MERL should be considered to be a useful potential therapeutic agent for use in gastric cancer treatment.